Sean Gilmore (15-LW-023)
Abstract
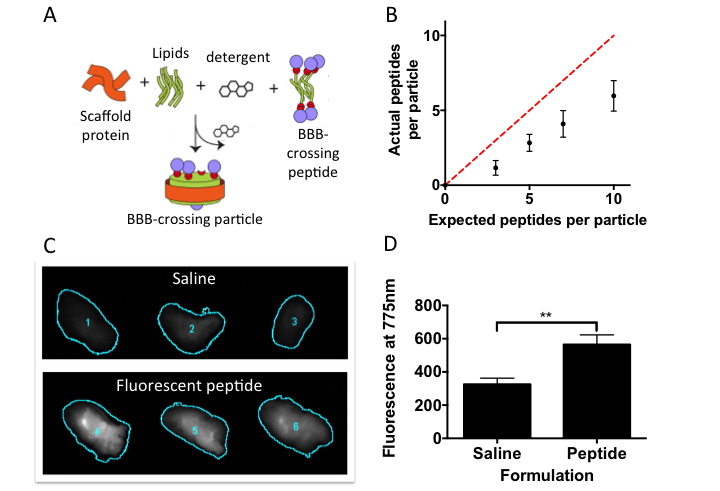
Therapeutic delivery to the brain is challenging because of the limited permeability of the blood–brain barrier, which impedes influx of most compounds from the blood to the brain. Traditional therapeutics often cannot permeate the barrier because it is composed of tightly apposed microvascular endothelial cells with specialized intercellular tight junctions, which severely limit trans-cellular traffic. A compound must satisfy a specific range of physical properties, including low molecular weight, high lipophilicity, and very low levels of serum protein binding to successfully cross the blood–brain barrier. Such restrictions drastically reduce the range of compounds with potential for sufficient biological availability in the brain. This is a considerable concern in the realm of drug design because a wide range of debilitating neurological conditions are in need of novel treatments. Many of these conditions are accompanied by exceptionally severe clinical consequences, including neurodegenerative disorders, brain tumors, bacterial or viral meningitis, and neurotoxin exposure. We propose that a nanometer-scale lipoprotein particle would be an ideal platform for integrating a wide range of therapeutic compounds with component ligands that promote active, efficient, and targeted delivery to the brain (shown in figure).
We expect to develop and optimize a novel nanoparticle platform for rapid and efficient delivery of a drug payload across the blood–brain barrier. This platform will incorporate therapeutically active compounds with ligands that trigger transport into the central nervous system. Such technology would meet an essential need for novel tools to improve efficacy of therapy relevant to the central nervous system via enhancement of blood–brain barrier permeability. This would lead to significant improvement of the standard of care throughout the field of neurobiology, yielding a tool that could provide protective capacity against many neurological diseases. We will assemble nanolipoproteins with targeting peptides and a fluorescent therapeutic surrogate. We will optimize the nanolipoprotein composition and targeting peptides through screening of a platform in an established model of the human blood–brain barrier. Then we will test top candidate particle compositions for in vivo biological availability to the central nervous system.
Mission Relevance
Our research addresses an important aspect of the Laboratory's core competency in bioscience and bioengineering relevant to developing advanced biological tools and platforms for medical countermeasures to biological or chemical agent threats. Development of a tool to deliver therapeutics to the central nervous system can safeguard military and civilian populations by providing a technology that could be utilized to increase survival and reduce pathology caused by neurological diseases.
FY15 Accomplishments and Results
In FY15 we (1) successfully formulated targeting peptides (TAT, G23, and AngioPep) with the nanolipoprotein platform; (2) successfully developed an in vitro blood–brain barrier model using human brain endothelial cells in combination with human astrocyte neurological cells; (3) demonstrated that this in vitro blood–brain barrier model is excellent for evaluating efflux and influx, and assessed the model's ability to evaluate transcytosis, which is the mode of blood–brain barrier crossing we are attempting to exploit; (4) completed most of the in vitro analysis; and (5) initiated the Laboratory's institutional animal care and use committee approval process for in vivo blood–brain barrier crossing experiments, which will begin in FY16.