Tony van Buuren (14-ERD-067)
Abstract
An operando x-ray diffraction system is presented for elucidating optimal laser-assisted chemical vapor deposition growth conditions for ultrahard materials. The technique is utilized to investigate deposition of crystalline silicon carbide (SiC) using trimethylsilane precursors and boron–carbon materials using trimethyl borate precursor. Trimethyl borate exhibits vastly reduced toxicological and flammability hazards compared to existing precursors but has previously not been applied to boron carbide growth. Crystalline boron-rich carbide material is produced on addition of hydrogen during the growth phase at high temperature. Crystalline SiC layers or amorphous SiC wires could be grown depending on the pressure of precursor. The use of the operando x-ray diffraction/absorption system allows for the exploration of highly non-equilibrium conditions and rapid process control, which are not possible using ex situ diagnostics.
Background and Research Objectives
Laser-assisted chemical vapor deposition (L-CVD) provides the means to deposit high quality, crystalline and conformal films with a high degree of spatial control. In the pyrolytic mode of L-CVD, selective deposition of material is achieved by thermally decomposing gaseous precursors using localized laser heating of a substrate (Ehrlich and Tsao 1983). The ability to control the location of the deposit enables the fabrication of films with complex structures, including materials with gradiated compositions (both laterally and vertically) and asymmetric properties (Lehmann and Stuke 1994; Wanke 1997; Yeo et al. 2013). Fully harnessing the capabilities of L-CVD requires a detailed understanding of the relationship between the structure/composition of the deposit and the process conditions. Identifying these relationships using ex situ, Edisonian, approaches is an arduous process, involving exploration of a matrix of numerous parameters that include the substrate temperature, laser profile, and gas compositions and pressure. The development of in situ/operando structural diagnostics is, therefore, essential for the rapid evaluation of the effects of varying deposition conditions; furthermore, operando methods also provide the potential for exploring highly non-equilibrium conditions and the possibility of real-time process control, which are not possible using ex situ diagnostics. In this paper, the development and use of a system for the operando x-ray diffraction (XRD) x-ray absorption (XAS) characterization of L-CVD grown films is reported. Boron and silicon carbide materials were selected in this study for two important reasons: (1) this class of materials exhibit ultra-hard and radiological properties that have a range of technological uses but are challenging to deposit via conventional methods, particularly with precise control over the location of the film (Suri et al. 2010), and (2) a potential new precursor gas, trimethyl borate (TMB, B(OCH3)3) (Sachdev et al. 2011; Muller et al. 2013) has been identified for CVD that is vastly less toxic and flammable than traditional precursors (such as BCl3 and B2H6 (Suri et al. 2010). The availability of a less hazardous precursor is significant because understanding the processes of boron carbide formation using TMB could lead to routine use that would enhance safety and, by extension, could improve the economics of boron-based coatings for numerous applications.
Scientific Approach and Accomplishments
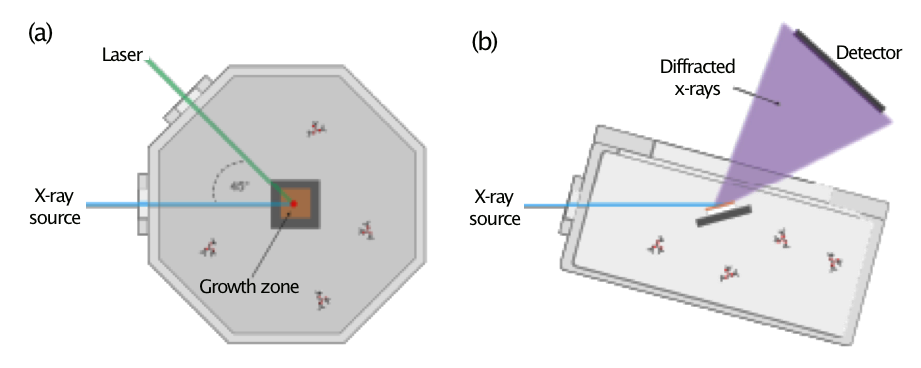
The operando XRD system for time-resolved measurement of crystalline material growth during L-CVD is based on a Kimball Physics 8” Spherical Square ultra-high-vacuum chamber. The chamber contains an optically transparent window for laser admittance and two beryllium windows for admittance and egress of x-rays (Figure 1). A 532-nm continuous-wave laser (5 W, Sprout-D, Lighthouse Photonics) was focused to a Gaussian beam radius of 475 µm and irradiated the substrate at a 75-degree angle of incidence to generate localized heat in the substrate. Mixtures of TMB and hydrogen were used as material growth precursors. Three temperature regimes were studied using varied substrate parameters to enable the heating profiles. Copper-coated (20 nm copper on 5 nm titanium) eight-percent yttria-stabilized zirconia (YSZ, MTI Corporation) substrates were used as the growth platform at low (2.63 W laser power) and intermediate (2.98 W laser power) temperatures as they provided effective coupling of the incident laser light to reach controlled temperatures. For the high-temperature regime, amorphous carbon-coated (20 nm) eight-percent yttria-stabilized zirconia substrates were used as the growth platform to provide maximum light coupling. To further increase the temperature, the system was also reconfigured to provide a laser angle of incidence of 10 degrees during these growths while maintaining a laser power of 2.98 W.
XRD measurements were performed on beamline 33-ID-D at the Advanced Photon Source (Argonne National Laboratory) using 8 kV x-rays (1.514 Å). Incident x-rays were aligned to be coincident with the laser on the sample surface to probe the L-CVD growth region and the diffracted x-ray signal was collected by a PilatusII 100K area detector. The x-ray beam spot was approximately 1,000 × 100 µm in size and irradiated a surface area of 1,000 × 386 µm at the 75-degree angle of incidence used during the experiments; the entire cross-section of the deposit was probed by XRD during growth. A well-defined procedure was performed via sequential steps for material growth and operandi measurement: (1) the vacuum chamber was pumped until high-vacuum was obtained; (2) TMB precursor and hydrogen (if required) were admitted into the vacuum chamber; (3) an initial XRD pattern of the substrate was collected; and (4) the laser was then turned on and sequential XRD patterns were collected until growth was halted by turning off the laser. Each XRD pattern collection in the low- and intermediate-temperature regimes required approximately four minutes, with the measurement sequentially acquired in the region where the majority of boron-carbon material diffraction peaks were anticipated.
Postmortem analysis of each deposit was performed ex situ using Raman and energy-dispersive x-ray spectroscopy (EDS) to determine their steady-state structure and atomic composition. Raman spectroscopy was performed using a Thermo Scientific Nicolet Almega XR micro-Raman system using a 633-nm, 1 mW laser and a 50-power magnification, 0.5 NA objective lens. EDS was performed using a Phenom ProX scanning electron microscope (SEM) at 5 keV electron energy.
As an initial stage of analysis, the XRD patterns were calibrated by a linear-fitting function using known YSZ peak positions to account for small misalignments in the experimental geometry before applying a Sonneveld-Visser baseline subtraction routine. The collected XRD spectra showed major diffraction lines attributed to YSZ and copper. This is advantageous for our measurement because boron-carbide species typically exhibit a number of diffraction peaks where there are minimal contributions from YSZ.
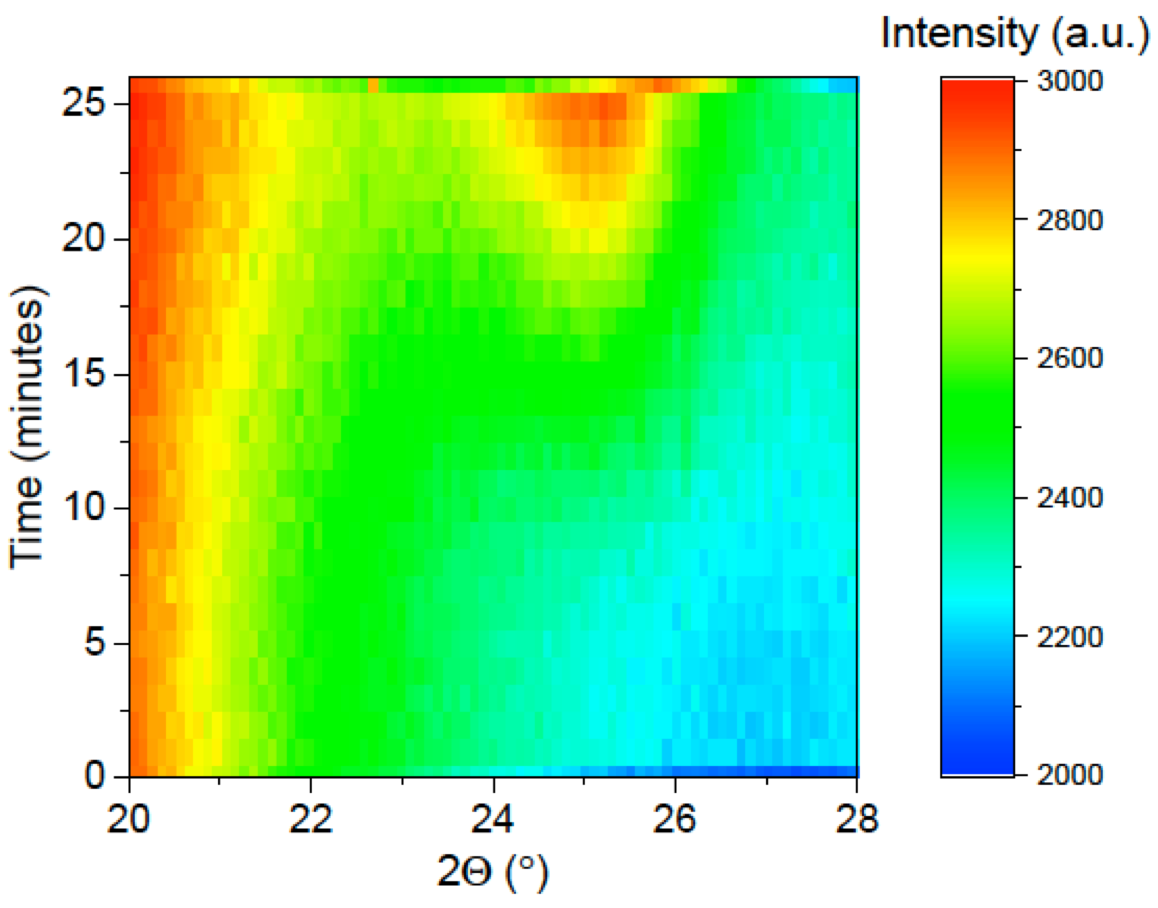
To determine the effectiveness of the technique to measure material growth with meaningful resolutions, initial L-CVD/XRD measurements were performed under conditions known to lead to deposition: 50 Torr of TMB and 50 Torr of hydrogen as the precursor material in the high-temperature regime (calculated temperature > 1,200 °C). Figure 2 shows a clear indication of the effectiveness of the technique as it displays the XRD patterns collected as a function of L-CVD growth time under these conditions. Growth of material is quickly detected, with a major amorphous diffraction peak appearing at 25 minutes, and no evidence of crystalline material growth during substrate heating or subsequent cooling.
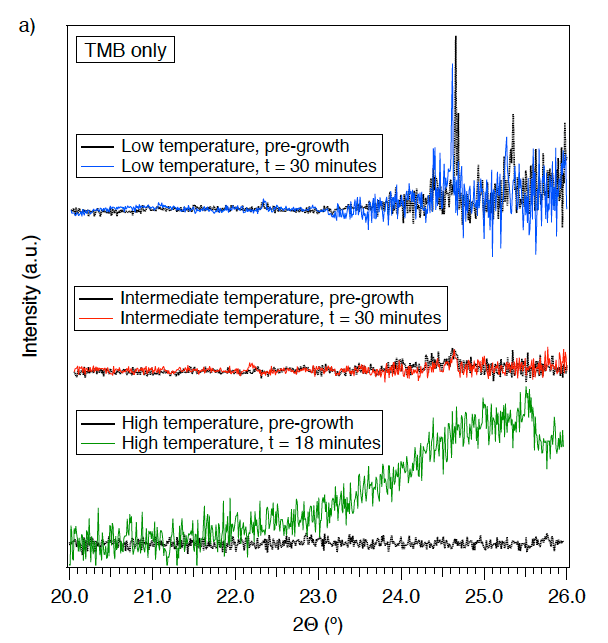
Additional operando measurements during L-CVD were performed to provide new insight into the material growth mechanism for boron carbides using TMB precursor, via variation in the heating conditions and the pressure and composition of the reactant gases. First, L-CVD was performed in the absence of hydrogen, using 10 Torr of TMB and a laser power of 2.63 W, and 20 Torr of TMB and a laser power of 2.98 W (Figure 3a).
Growth of crystalline material is not evident in the XRD pattern and the cause is ascribed to deposition of amorphous material containing boron, oxygen and/or carbon in the gradated heating zone. This is consistent with other systems (such as diamond) where hydrogen drives crystallization through removal of deposited amorphous phases, activation of reaction pathways between the pre-cursor and substrate, and reaction between precursor and hydrogen in the gas phase (Matsui, and Sahara 1989; Frenklach and Wang, 1991).
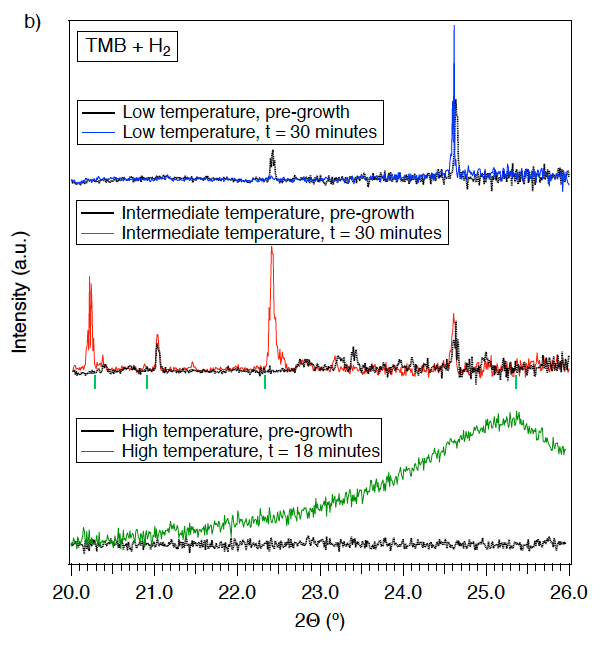
Depositions with hydrogen were performed using 10 Torr of TMB and 42 Torr of hydrogen as the precursor material, but with varying laser powers (2.63 and 2.98 W, respectively) (Figure 3b). Growth of crystalline material was not observed at a laser power of 2.63 W. A transition in growth profiles was observed upon increasing the laser power from 2.63 to 2.98 W (peak temperature of approximately 680 to 770 ºC), resulting in growth of crystalline material. Growth of crystalline material was quickly detected with major diffraction peaks appearing at 20.22 and 22.41 degrees and minor peaks at 20.38, 20.88, 21.46 and 22.55 degrees. XRD peaks observed in the material during growth are consistent with the deposition of a boron-rich carbide material, with B8C (Plug 1974) providing the closest match to peaks observed over the range measured. Other materials composed of elements present in the reaction environment that could lead to peaks in the 20–26 degree range (such as boron oxides) did not provide as close a match as B8C; moreover, deposits of these materials are generally amorphous and occur in the crystalline phase only under high-pressure/high-temperature conditions. B (Callmer 1976), B11.4C3.6 (Konovalikhin and Ponomarev 2009), B4C (Clark and Hoard 1943), and B10.4C1.6 (Konovalikhin and Ponomarev 2009) were also considered; however, these did not represent a good match to the collected data. The assignment of B8C is significant because it is a metastable material (Amberger et al. 1981; Velamakanni et al. 2009) that would not be observed ex situ. This clearly demonstrates that the operando XRD measurement system is ideal for the study of L-CVD growth under thermodynamically challenging and non-equilibrium conditions. Further studies using the operando XRD approach could probe thermodynamic and kinetic properties of metastable materials where the system undergoes a phase or composition change during growth, which could yield identification of new structures at high temperature. As shown here, it can be harnessed to promptly find these conditions in a previously unexplored system. We noted that the deposit was not pure stoichiometric B8C throughout its entire lateral cross-section due to thermal gradients induced by laser heating; nonetheless, the non-uniform heating allows for material growth under a range of temperatures within the deposit that can be probed simultaneously via XRD. Laser heating therefore allows rapid probing of a new precursor to determine if favorable conditions are present anywhere in the system.
Finally, depositions were performed using 50 Torr TMB with and without the presence of 50 Torr of hydrogen with a laser power of 2.98 W and a 10-degree angle of incidence. In this high-temperature (> 1,200 ºC) growth regime, both deposits were amorphous in structure, with broad features centered around 25 degrees in the collected XRD pattern. This regime also brought about notable changes to the YSZ substrate that were not observed in the other growth regimes. This enabled the experimental determination of a minimum substrate temperature based on the shift in the YSZ substrate peak position.
Postmortem analysis of the deposits revealed interesting differences between the deposits fabricated at intermediate and high temperatures. Raman spectroscopy indicates a mixture of single crystal and amorphous material at the intermediate growth regime with the addition of hydrogen while deposits formed in the high-temperature regime display graphitic carbon characteristics. Analysis of the material by EDS shows further differences between the two growth regimes. The intermediate growth deposit contains boron, carbon, and oxygen, while the high-temperature-regime deposit only contains carbon. The surface temperature in the high-temperature regime was therefore hot enough to efficiently desorb boron and oxygen from the surface.
Impact on Mission
Our new L-CVD capability and in situ diagnostics expertise supports Lawrence Livermore National Laboratory's advanced materials and manufacturing core competency, especially with regard to development of new materials and partnership building.
Conclusion
Operando XRD measurements were used to characterize L-CVD material using a TMB precursor under varied process conditions. The presence of hydrogen at elevated temperature was shown to be critical in the production of boron-rich carbide material. The ability to observe reactions during operation allows the characterization of transient and metastable phases that would not be observed ex situ and limits the need for arduous processing routines. These results demonstrated a platform for accelerated implementation of novel chemical vapor deposition systems and a method for process control under conventional growth recipes.
References
Amberger, E. et al. 1981. "Gmelin Handbook of Inorganic Chemistry." Springer-Verlag, Berlin.
Callmer, B. 1976. "An Accurate Refinement of the β-Rhombohedral Boron Structure." Acta Crystallographica B33: 1951–1954.
Clark, H. K. and J. L. Hoard. 1943. "The Crystal Structure of Boron Carbide." Journal of the American Chemical Society 65 (11): 2115–2119. doi: 10.1021/ja01251a026.
Coker, E. N., et al. 2012. “Using in-situ Techniques to Probe High-Temperature Reactions: Thermochemical Cycles for the Production of Synthetic Fuels from CO2 and Water.” Powder Diffraction 27 (2): 117–125. doi: 10.1017/S0885715612000255.
Ehrlich, D. J. and J. Y. Tsao. 1983. “A Review of Laser-Microchemical Processing.” Journal of Vacuum Science & Technology B, Nanotechnology and Microelectronics: Materials, Processing, Measurement, and Phenomena 1: 969. doi: 10.1116/1.582718.
Fawcett, T. G., et al. "PDF-4+, the Material Identification Database." Microstructure Analysis in Materials Science, Freiberg, June 15–17 2005.
Frenklach, M. and H. Wang. 1991. "Detailed Surface and Gas-Phase Chemical Kinetics of Diamond Deposition." Physical Review B 43: 1520–1545.
Konovalikhin, S. V. and V. I. Ponomarev. 2009. "Carbon in Boron Carbide: The Crystal Structure of B11.4C3.6." Russian Journal of Inorganic Chemistry 54(2):197–203. doi: 10.1134/S0036023609020053.
Langford, J. I. and A. J. C. Wilson. 1978. “Scherrer After Sixty Years: A Survey and Some New Results in the Determination of Crystallite Size.” Journal of Applied Crystallography 11(2):102–113. doi: 10.1107/S0021889878012844.
Lehmann, O. and M. Stuke. 1994. “Three-Dimensional Laser Direct Writing of Electrically Conducting and Isolating Microstructures.” Materials Letters 21 (2): 131—136. doi: 10.1016/0167-577X(94)90206-2.
Matsui, Y. and M. Sahara. 1989. "Chemical Kinetic Analysis on the Growth Mechanism of Diamondlike Films from a CH3OH-H2 Mixture." Japanese Journal of Applied Physics 28, Part 1 (6).
Matthews, M., et al. 2013. “Localized Planarization of Optical Damage Using Laser-Based Chemical Vapor Deposition.” Proceedings of SPIE: 8885. doi: 10.1117/12.2030506.
Muller, F., et al. 2013. "Increasing the Wear Resistance by Interstitial Alloying with Boron via Chemical Vapor Deposition." Langmuir 29 (14): 4543–4553. doi: 10.1021/la400148h.
Plug, K. 1974. “Composition and Structure of Boron Carbides Prepared by CVD.” Journal of Crystal Growth 24-25 (C):197–204.
Sachdev, H., et al. 2011. “Formation of Boron-Based Films and Boron Nitride Layers by CVD of a Boron Ester.” Angewandte Chemie 50 (16): 3701–3705. doi: 10.1002/anie.201003012.
Suri, A. K., et al. 2010. “Synthesis and Consolidation of Boron Carbide: A Review.” International Materials Reviews 55(1): 4–38. doi: 10.1179/095066009X12506721665211.
Velamakanni, A., et al. 2009. "Catalyst-Free Synthesis and Characterization of Metastable Boron Carbide Nanowires." Advanced Functional Materials 19 (24): 3926–3933.
Wanke, M. C. 1997. “Laser Rapid Prototyping of Photonic Band-Gap Microstructures.” Science 275 (5304): 1284–1286. doi: 10.1126/science.275.5304.1284.
Yeo, J., et al. 2013. “Rapid, One-Step, Digital Selective Growth of ZnO Nanowires on 3D Structures Using Laser Induced Hydrothermal Growth.” Advanced Functional Materials 23 (26): 3316—3323. doi: 10.1002/adfm.201203863.
Publications and Presentations
Bagge-Hansen, M., et al. 2016. "In Situ Studies of Nanoporous Materials for Energy Storage and Conversion." SSRL/LCLS Annual Users’ Meeting and Workshops. LLNL-PRES-704390.
DePond, P., et al. 2016a. "Probing CVD Growth Mechanisms of SiC with In Operando Synchrotron Based X-ray Diagnostics." 2016 43rd International Conference on Metallurgical Coatings and Thin Films, San Diego, CA, 25-29 April 2016. LLNL-PRES-690661.
——— 2016b. "Probing CVD Growth Mechanisms of SiC." AVS 63rd International Symposium and Exhibition, Nashville, TN, 6 November 2016. LLNL-PRES-708400.
Lee, J. R. I., et al. 2016. "In Situ X-ray Diffraction of Laser Chemical Vapor Deposition Ultra-Hard Coatings." In situ X-ray characterization of Selective Laser Melting (SLM) Additive Manufacturing Processes for Energy Applications. LLNL-PROP-726825.
——— 2017. "In Situ X-ray Diffraction of Laser Chemical Vapor Deposition Ultra-Hard Coatings." ALS-CXRO Materials Science Seminar. LLNL-PROP-726825.
Van Buuren, A. 2016. "Structure of Carbon Nanotube Porins in Lipid Bilayers: An In Situ Small-Angle X-ray Scattering (SAXS) Study." Nano Letters 16, 4019. LLNL-JRNL-679134.