Carlos Valdez (14-ERD-048)
Abstract
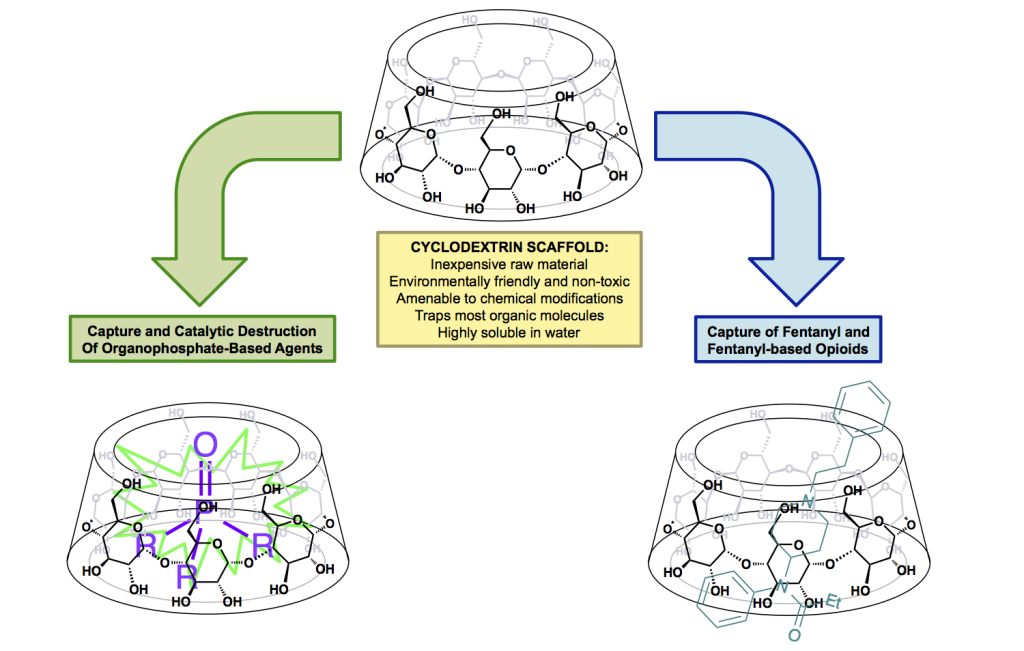
New technologies for capturing and catalytically degrading chemical weapon agents would fill a critical national security need. We address the use of molecular-complex scaffolds known as cyclodextrins that possess chemical and physical characteristics suitable for detection and analysis, decontamination, and medical countermeasures where the demand for broad-spectrum solutions is urgent. Our research project focuses on efforts within two classes of chemical warfare agents: the organophosphorus-based nerve agents and a series of incapacitating agents known as fentanyls. Both are of high priority because of their ease of production and availability and their toxicity at low doses. We intend to develop and validate an integrated experimental and computational approach whose goal is the development of cyclodextrin-based nanometer-scale scaffolds for the capture and catalytic destruction of organophosphorus nerve agents and fentanyls. Successful technologies have been developed to mitigate individual issues for known chemical threats. However, establishing broadly applicable and rapidly responsive methodologies targeted at known and (more importantly) emerging threats, remains critical. Although our research addresses the development of designer cyclodextrin molecules for two classes of agents, the foundation of our work is an integrated experimental and computational strategy for the discovery of efficacious cyclodextrins for broad-spectrum application.
We expect to develop an understanding of the mechanism of action of fentanyl on protein receptors, which is key to the development of cyclodextrin for sequestration of these toxic chemicals. Elucidating conditions under which zinc-based organometallic catalysts most efficiently degrade organophosphorus agents will provide insight into the development of effective metallocyclodextrins for decontamination. While the cyclodextrins themselves are important for mitigating organophosphorus- and fentanyl-specific threats, it is the development, demonstration, and validation of an integrated design approach capable of optimizing arbitrary host–guest complexes that will be the central accomplishment of our research.
Mission Relevance
Our strategy focuses on the development of physical and medical countermeasures against fentanyls and organophosphorus nerve agents, which supports the Laboratory's strategic focus area in chemical and biological security for the rapid mitigation of evolving and unknown threats. This project also supports the LLNL core competency in bioscience and bioengineering by evolving an integrated computational and experimental capability for the rapid discovery of highly effective, broadly applicable scaffolds. Through this effort, we are providing a foundation for future progress in environmental remediation technologies, exposure detection capabilities, and the production of advanced materials for use against known and emerging threats.
FY15 Accomplishments and Results
In FY15 we (1) synthesized nine fentanyls for our studies using an improved synthetic route developed in our laboratory, (2) evaluated four zinc-based catalysts in the hydrolysis of the organophosphate-based pesticide paraoxon, (3) evaluated the ability of several cyclodextrins to bind with all nine of the fentanyl compounds we synthesized, and (4) gathered data on the binding of paraoxon (an organophosphate insecticide), along with its hydrolysis products, to b-cyclodextrin.
Publications and Presentations
- Hok, S., et al., Improved and optimized syntheses of fentanyl and related analogs. (2015). LLNL-POST-675520.
- Mayer, B. P., S. E. Baker, and C. A. Valdez, “Kinetics and speciation of paraoxon hydrolysis by zinc(II)-azamacrocyclic catalysts.” Inorg. Chim. Acta. 436, 123 (2015). LLNL-JRNL-663756.