Sean Gilmore (15-LW-023)
Abstract
The blood–brain barrier protects the brain from foreign or harmful material, but also blocks access of many potentially therapeutic molecules that could be used to treat conditions of the central nervous system. Traditional therapeutics often cannot permeate the barrier because it is composed of tightly apposed microvascular endothelial cells with specialized intercellular tight junctions, which severely limit trans-cellular traffic. However, it is known that cells comprising the barrier can transport molecules such as apoA1, a component of high-density lipoprotein particles, into the brain. Our goal was to develop a synthetic nanoparticle that can be transported into the brain by mimicking apoA1 high-density lipoproteins. We demonstrated that these particles can be conjugated with small molecules that served as surrogate therapeutic cargo. The ability of these particles to cross the blood–brain barrier was evaluated in both an in vitro cell model of the barrier and Sprague–Dawley rats. The in vivo rat model showed that apoA1-derived nanolipoprotein particles with surrogate therapeutic cargo can cross the blood–brain barrier and are detectable in the brain 24 hours following injection. Further quantification and in-depth analysis of nanoparticle crossing of the barrier will be achieved by accelerator mass spectrometry initiated under this project.
Background and Research Objectives
Treating conditions of the central nervous system, such as gliomas (tumors of the brain’s supportive tissue), viral infections, or nerve agent exposure, can be challenging because potentially therapeutic compounds may not be able to access the brain while circulating in blood. This is because of the blood–brain barrier, which restricts entry into the brain, thereby protecting it from infection or potentially harmful chemicals. The barrier itself is composed of vascular cells that connect to each other at tight junctions that prevent even small molecules from diffusing into the brain. While many molecules or compounds that are essential to the brain are able to diffuse across this barrier, it often excludes therapeutics because of their physical and chemical properties. Unfortunately, modifying therapeutics to enable diffusion across the blood–brain barrier is very difficult, and may not be possible for larger compounds, such as biologics.
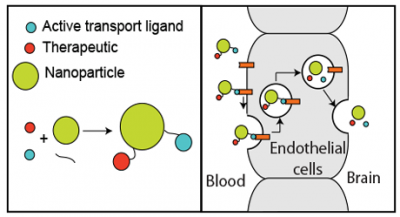
In order to address this problem, it would be desirable to develop a delivery particle capable of entering the brain from circulating blood, while being able to shuttle a wide variety of different types of therapeutics (Figure 1). Additionally, these particles would need to be stable while circulating in blood for at least several hours, because dissolution of the particle structure would prevent the cargo from being transported across the blood–brain barrier. In order to accomplish this, it would be advantageous to exploit the cells that comprise the barrier themselves because they actively transport some material into the brain. In particular, it is known that specific types of high-density lipoproteins (apolipoprotein A1, or apoA1) can be detected in cerebrospinal fluid, but are synthesized elsewhere in the body and are transported into the brain as described by Vitali.1 Further, as demonstrated by the work of Stukas,2 recombinant apoA1 can be detected in the brain as soon as 30 minutes after injection. With this in mind, we have developed a synthetic nanoparticle that closely mimics those that are actively transported into the brain. These high-density lipoprotein mimetics, termed nanolipoprotein particles, were functionalized in a variety of ways, including chemically crosslinking the internal structure, which enabled the particles to be stable for hours while circulating in blood. Additionally, the particles were loaded with fluorescent molecules to serve as surrogate therapeutic small molecules while also facilitating the quantitative measurements of the particles in biological media. Finally, we conjugated the peptide G23 to the particles with the goal of further enhancing their rate of transport into the brain. This peptide has been reported by Georgieva3 to enable transport across the blood–brain barrier both in vitro and in vivo.
Scientific Approach and Accomplishments
Preparation of a Surrogate Therapeutic: DOPE-Cy7
The first step to evaluating nanoparticle shuttling across the blood–brain barrier was to fluorescently label the nanoparticles, demonstrating the feasibility of incorporating small molecules into the nanoparticle (e.g., therapeutics) as well as providing an optical marker for quantifying the nanoparticle and their in vivo localization. To fluorescently label the particles, we selected the infrared fluorophore Cy7, which was covalently conjugated to the phospholipid DOPE and subsequently purified using reversed-phase high-performance liquid chromatography. Collected fractions were lyophilized, and the material was solubilized in methanol and quantified using a spectrophotometer (Nanodrop ND-1000, ThermoScientific, Lafayette, Colorado). We were able to incorporate DOPE-Cy7 into the lipid matrix of the nanolipoprotein particles up to 15 mol%. At higher mole percent, we observed that the particles became unstable during assembly and purification.
Nanolipoprotein Particle Synthesis
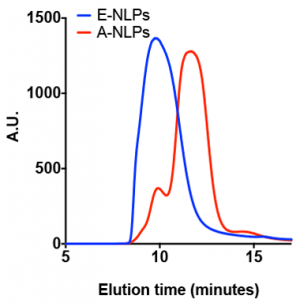
As mentioned, we aimed to demonstrate that nanolipoprotein particles constructed with apolipoprotein A derivatives are capable of crossing the blood–brain barrier. Conversely, other apolipoprotein particles, such as those with apolipoprotein E, are not able to cross the barrier in vivo, as reported by Liu.4 Two types of nanolipoprotein particles were assembled using either apolipoprotein nanolipoprotein A derivatives (A-NLPs) or apolipoprotein E derivatives (E-NLPs). In these experiments, the E-NLPs served two purposes. First, they allowed us to use a similar construct as a negative control, and second, we assessed the ability of G23 to enable blood–brain barrier transport. Nanolipoprotein particles were assembled according to procedures previously reported by Blanchette et al.5–9 with slight modifications. Dried lipid films were solubilized in a PBS (phosphate-buffered saline) buffer with sodium cholate. After addition of the scaffold protein, samples were incubated at 35°C for 30 min. and then cooled to 25°C for 90 min. The lipid–protein solution was then mixed with BioRad BioBeads for 2 h to remove the cholate, enabling self-assembly of the nanolipoprotein particles. The particles were labeled with either AF488 by incubating them with the reactive dye for at least 2 h. Samples were subsequently analyzed and purified by size exclusion chromatography (Figure 2). Chromatography fractions (500 µl) were collected every 30 s. Fractions containing homogeneous nanolipoprotein particle populations were concentrated, and the nanolipoprotein particle concentrations were determined by using using reversed-phase high-performance liquid chromatography with standard curves for the separated scaffold proteins. The concentrated nanolipoprotein particle samples were then stored at 4°C until further use. The G23 peptide was obtained from GenScript (Piscataway, New Jersey), which came functionalized with an azide group. Conjugation of G23 to the nanolipoprotein particles was achieved by incorporating a lipid with a dibenzocyclooctyl (DBCO) reagent functional group attached to the head group with PEG2000. Conjugation reactions were allowed to take place overnight. Excess PEG7-azide was added to solution to react any remaining DBCO groups on the particles.
Cell Methods and Experiments
To screen a wide variety of both A- and E-NLP formulations, we used a cell model of the blood–brain barrier. To establish this model, we chose the human blood–brain barrier cell line hcMEC/D3 obtained from ATCC, the global biological materials resource and standards organization, and cultured as recommended. Once confluent, cells were plated onto transwell plates with a polyester membrane featuring 0.4-μm pores. Once plated, cells were monitored every other day, and the transendothelial electrical resistance was measured and recorded. The transendothelial electrical resistance value is commonly used as a metric for the impermeability of the cell monolayer grown on transwell membranes. For all experiments, cells were grown until a minimum transendothelial electrical resistance value of 30 Ω × cm2 was obtained.
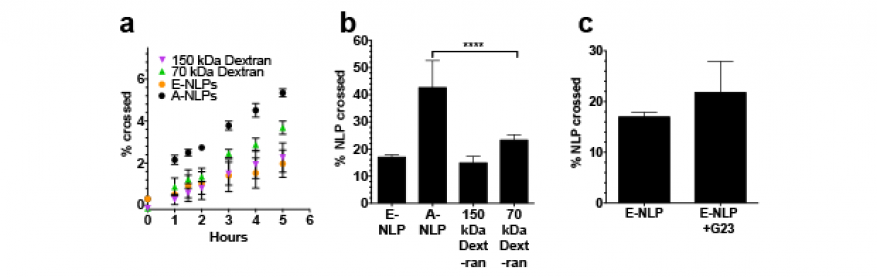
Cells were dosed in the apical compartment of the transwell plates with 10 μg of nanolipoprotein particle material (by scaffold protein mass). As a control, we used an equivalent mass of fluorescently labeled dextrans that are not actively transported across the cell monolayer, nor are they able to diffuse through the cells. The media in the basal compartment were then sampled over time (50-μl volumes) and analyzed using a fluorescence plate reader (BioTek, Winooski, Vermont). We observed that there was little difference between the rate at which E-NLPs cross the cell layer versus the rate at which the dextran controls cross (Figure 3). However, we do observe that the A-NLPs cross slightly faster even at short time points, and at 24-h time points, we found that the A-NLPs had the fastest rate of transport (p < 0.00001). As an additional test, we compared nanolipoprotein particles that were conjugated with G23 peptide (approximately 20 peptides per particle) to those without, shown in Figure 3(c). We observed a marginal, but not significant increase in the rate of transport of these nanolipoprotein particle formulations across the cell monolayer.
These experiments show that the hcMEC/D3 transwell monolayer is an imperfect model for simulating the blood–brain barrier because it does allow passive diffusion through the transwell membrane, even for molecules as large as 150 kDa. We found that the rate of passive diffusion of the dextrans is comparable to the rate of diffusion of the E-NLPs. However, we do observe that A-NLPs exhibit faster transport than even the 70-kDa dextran and E-NLPs at 24 h. This finding is consistent with the prediction that apolipoprotein A-derived particles will be actively transported across the blood–brain barrier, which accounts for the enhanced rate of transport. When we conjugated the G23 peptide to our nanolipoprotein particle, we were not able to enhance the rate of transport of the particles across the monolayer. However, it is possible that this finding is a result of the limitations of the cell model of the barrier.
Animal Methods and Experiments
While the hcMEC model is widely used to study transport of material across the blood–brain barrier, in vivo models remain the gold standard for studying blood–brain barrier transport or permeability. Evaluation of nanolipoprotein particles in animals was performed both to characterize the behavior of the particles in circulating blood, and to assess particle transport into the brain. The ultimate goal of these tests was to detect the presence of A-NLPs in the brain, ideally even after they had been eliminated from the blood. Experiments were performed in male Sprague–Dawley rats, aged 8 to 10 weeks and weighing approximately 250 to 300 g. To minimize dosing variability and to facilitate blood collection, we implanted a jugular vein catheter that was accessible on the back of the rats.
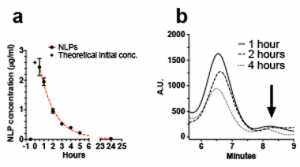
Rats were dosed with 40 μg of A- or E-NLPs/250 g of body weight, with nanolipoprotein particle solutions being prepared at a concentration of 0.1 μg/μl in saline solution. A control group of animals was injected with an equivalent amount of Cy7-labeled liposomes. In blood clearance experiments, blood was collected prior to injection, and then at 30 min, hourly from 1 to 5 h, and at 24 h (Figure 4). Whole blood was analyzed using a fluorescence plate reader, and blood serum was analyzed by size-exclusion chromatography to confirm that the observed signal was attributable to intact nanolipoprotein particles, because intact particles elute at a different time compared to the free scaffold protein. This detail is an essential point in our drug-delivery scheme: if the nanolipoprotein particles were to dissociate in circulation, then the cargo would not be able to be delivered into the brain.
Analysis of the whole blood revealed that nanolipoprotein particles are eventually cleared from the blood over several hours. At the first post-injection blood draw, almost all of the particles are still circulating. Between 1 and 2 h, 50% of the nanolipoprotein particles are still in circulation, but by 24 h, the particles are no longer detectable in sampled blood. In the analyzed serum, we were able to clearly identify peaks associated with intact particles (6–7 min for A-NLPs). The magnitude of these peaks was observed to diminish over the sampled time range of 1 to 4 h. Interestingly, we did not detect an increasing signal from the free scaffold protein (indicated by arrow), which would be expected if the particles were dissociating. This finding suggests that either the particles are cleared from circulation while intact, or the free apolipoprotein itself is rapidly cleared from blood. It is also possible that it re-associates with nascent high-density lipoproteins, but additional work is needed to confirm these scenarios.
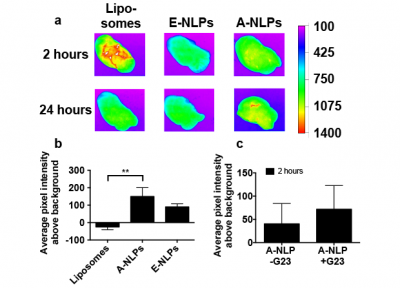
After characterizing the behavior of the nanolipoprotein particles in blood, we focused on analyzing the brains for fluorescent signal. At a chosen interval after dosing, animals were perfused with a heparin solution to remove all blood in the brain capillaries. This is especially important, since the fluorescent particles could still be in circulation, creating an artificially high signal in the brain. After completing the perfusion process, cerebrospinal fluid was collected as described by Nirogi,10 and organs including heart, lungs, liver, spleen, kidneys, and brain were collected for analysis. Brains were imaged using a Kodak fluorescence imager (Figure 5). Brains from negative control animals were imaged to obtain an average background pixel value.
At 2 h, we detected some area of enhanced fluorescent intensity in brains from animals injected with the A-NLPs, but a negligible signal in the E-NLP group, shown in Figure 5(a). Surprisingly, we also saw a large amount of signal in the brains of animals dosed with the liposomes. This is likely because of incomplete perfusion of the liposomal material from the brain vasculature. Regardless, we did not observe any measurable signal over background in the brains of the control group at 24 h, which is consistent with our measurements showing that liposomes are fully cleared from circulation after 24 h. Again, we saw only marginal signal above background in the E-NLP group, but in the A-NLP group we found a significant amount of signal above our control, shown in Figure 5(b). This is an especially exciting finding given that the blood concentration of A-NLPs at 24 h is negligible. We then aimed to enhance signal detected in the brain by conjugating G23 to the particles. Again, as with the in vitro experiments, we saw a measurable increase in signal, shown in Figure 5(c), although additional experiments would be required to verify the statistical significance of this finding.
While fluorescence in the brain was detected in the A-NLP group, we have not been successful in detecting any fluorescence from the particles in the cerebrospinal fluid collected thus far. To overcome the limited sensitivity of fluorescence techniques, we developed protocols for assessing nanolipoprotein particle accumulation in the brain using accelerator mass spectrometry as a means of detecting our particles. This technique can achieve attamole sensitivity, which should allow us to verify that G23 is improving uptake into the brain. Accelerator mass spectrometry experiments were performed with nanolipoprotein particles containing radiolabeled tracers, and samples are being analyzed under a pilot program with the BioAMS facility at LLNL.
Impact on Mission
Our research addresses an important aspect of the Laboratory's core competency in bioscience and bioengineering relevant to developing advanced biological tools and platforms for medical countermeasures to biological or chemical agent threats. Lawrence Livermore has several active nanolipoprotein-particle projects, including vaccine development, protein structural studies, and more recently, as an agent for in vivo delivery of therapeutics. The technical achievements we have made (i.e., increasing stability of these particles in biological media) can be utilized by these or future nanolipoprotein-particle projects. One area of significant interest is the development of more effective therapeutics to treat exposure to nerve agents. The nanoparticle platform that we have developed is a promising candidate technology to deliver nerve agent therapeutics, such as acetylcholineesterase reactivators, to the brain. This is particularly critical, because current nerve agent countermeasures do not cross the blood–brain barrier, and thus are not protective in the central nervous system. Our observation that particles may be retained in the brain despite clearance from circulation raises therapeutic possibilities across a spectrum of neurological injuries and diseases relevant to biosecurity and national defense.
Conclusion
Under this LDRD project, we developed a long-circulating, bio-mimetic nanometer-scale particle capable of transporting material across the blood–brain barrier. We have established an experimental pipeline whereby we can readily develop novel nanoparticle formulations, evaluate them in vivo, and quantify compound accumulation in the central nervous system. In addition, our project partially funded a new postdoctoral researcher who has contributed expertise in the areas of amphiphile, a chemical compound possessing both hydrophilic (water-loving, polar) and lipophilic (fat-loving) properties; materials; and self-assembly techniques. As described previously, our nanolipoprotein particle technology has applications in treating a wide array of conditions of the central nervous system. In particular, and in keeping with the mission objectives of the Laboratory, we plan to apply this research to the development of formulations for the treatment of exposure to neurotoxins.
References
- Vitali, C., et al., “HDL and cholesterol handling in the brain.” Res. 103, 405 (2014). https://academic.oup.com/cardiovascres/article/103/3/405/2931058
- Stukas, S., et al., “Intravenously injected human apolipoprotein A-I rapidly enters the central nervous system via the choroid plexus.” Am. Heart Assoc. 3, 1 (2014).
- Georgieva, J. V., et al., “Peptide-mediated blood–brain barrier transport of polymersomes.” Chem. Int. Ed. 51, 8339 (2012).
- Liu, J. V., et al., “Apolipoprotein E does not cross the blood-cerebrospinal fluid barrier, as revealed by an improved technique for sampling CSF from mice.” J. Physiol. Regul. Integr. Com. Physiol. strong303(9), R903 (2012).
- Blanchette, C. D., et al., “Quantifying size distributions of nanolipoprotein particles with single-particle analysis and molecular dynamic simulations.” Lipid Res. 49, 1420 (2008). http://dx.doi.org/10.1194/jlr.M700586-JLR200
- Chromy, B. A., et al., “Different apolipoproteins impact nanolipoprotein particle formation.” Am. Chem. Soc. 129(46), 14348 (2007). UCRL-JRNL-233234. http://dx.doi.org/10.1021/ja074753y
- Fischer, N. O., et al., “Isolation, characterization, and stability of discretely-sized nanolipoprotein particles assembled with apolipophorin-III.” PLoS One 5(7), e11643 (2010). http://dx.doi.org/10.1371/journal.pone.0011643
- Fischer, N. O., et al., “Evaluation of nanolipoprotein particles (NLPs) as an in vivo delivery platform,” PLoS One 9(3), e93342 (2013). LLNL-JRNL-641712. http://dx.doi.org/10.1371/journal.pone.0093342
- Gilmore, S. F., et al., “Lipid cross-linking of nanolipoprotein particles substantially enhances serum stability and cellular uptake.” ACS App. Mat. Interfac. 8(32), 20549 (2016). http://dx.doi.org/10.1021/acsami.6b04609
- Nirogi, R., et al., “A simple and rapid method to collect the cerebrospinal fluid of rats and its application for the assessment of drug penetration into the central nervous system.” Neurosci. Meth. 178, 116 (2009).
Publications and Presentations
- Gilmore, S. F., et al., Evaluation of nanolipoprotein particles (NLPs) as an in vivo delivery platform for biomedical applications. (2016). LLNL-PRES-700839.
- Sean Gilmore, S., et al., “Lipid cross-linking of nanolipoprotein particles substantially enhances serum stability and cellular uptake.” ACS Appl. Mater. Inter. 8, 20549 (2016). LLNL-JRNL-683565. http://dx.doi.org/10.1021/acsami.6b04609