Sarah Baker | 17-FS-027
Overview
The goal of this project was to explore the feasibility of using advanced manufacturing (AM) to reinvent the microbes, particularly methane-consuming microbes (methanotrophs) used in industrial biocatalysis. We demonstrated that methanotrophs could be immobilized in ultraviolet-light-curable hydrogel scaffolds while maintaining cell activity. To increase the mechanical stability and permeability of the hydrogels, we developed two structural scaffolds for the immobilized cells, based on porous silicone and three-dimensionally printed acrylate. We demonstrated that the cells were usable in highly concentrated suspensions, excreted valuable products such as muconic acid, and maintained activity over several days.
Background and Research Objectives
A technology to convert methane efficiently to other hydrocarbons is highly sought as a profitable way to convert “stranded” sources of methane and natural gas (sources that are small, temporary, or distant from a pipeline) to liquids for further processing. Biological methane conversion requires significantly lower energy and capital costs than chemical conversion; however, current stirred-tank bioreactors are limited by the mass transfer of gas-phase reactants to biocatalysts. Lawrence Livermore National Laboratory (LLNL) has developed AM bioreactor materials that consist of printed enzyme-embedded polymers to intensify biological methane conversion reactions by increasing transfer and biocatalyst loading (Blanchette et al. 2016).
The enzyme-based process was not economical, however, because of the expensive reducing equivalents required to convert methane to methanol and poor enzyme stability over time. We explored whether live microbes, which manufacture their own reducing equivalents and can be significantly more stable, could be immobilized and structured using additive manufacturing with improved activity. We developed a partnership with the National Renewal Energy Laboratory (NREL) to undertake this study, as they had a team conducting a complementary project on engineering microbes (based on Methylococcus capsulatus ) to excrete valuable products from methane (Henard et al. 2016).
Our research objectives were to (1) encapsulate methanotroph cells in polymer materials and demonstrate catalytic activity and product excretion; (2) assess the catalytic activity of the immobilized cell material with and without an exogenous reducing agent; (3) tune material properties such as chemistry and geometry to increase volumetric productivity, methane diffusion, and product diffusion; and (4) determine the stability of the immobilized biocatalyst over time.
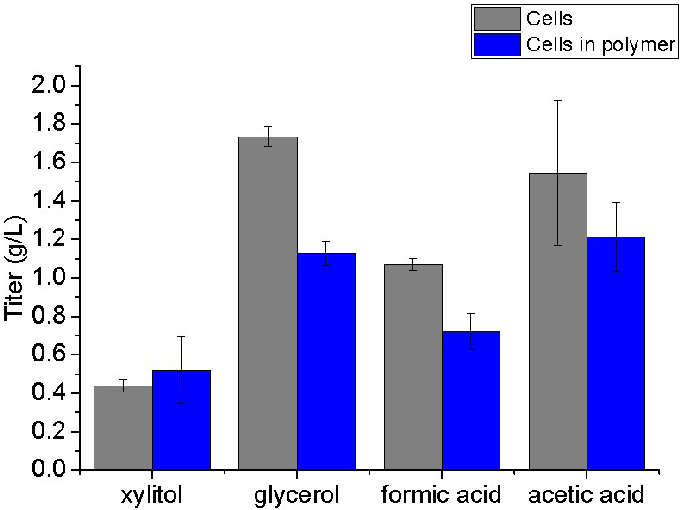
Recoverable products excreted by immobilized methanotroph cells are shown in Figure 1.
Impact on Mission
This project supports the DOE goal of supporting a an economically competitive, environmentally responsible, secure and resilient energy infrastructure. It is directly related to the Laboratory’s research challenge in energy and resource security and will help LLNL maintain a role in natural gas conversion technologies. This role is of great interest to LLNL because natural gas will likely be a major source of domestic energy in the future. Natural gas and methane can also be a bridge to renewables; for example, methane can be harvested from waste and other renewable biological sources and used for energy storage. This research also supports LLNL's core competency in advanced materials and manufacturing. This project facilitated a new and fruitful partnership with NREL resulting in joint proposals and new DOE funding to LLNL.
References
Blanchette, C. D., et al., "Printable Enzyme-Embedded Materials for Methane to Methanol Conversion," Nature Communications 7 (June 15, 2016): 11900. doi:10.1038/ncomms11900.
Henard, C. A., et al., "Bioconversion of Methane to Lactate by an Obligate Methanotrophic Bacterium," Scientific Report 6 (February 23, 2016): 21585. doi: 10.1038/srep21585.
Publications and Presentations
Baker, S., et al. 2018. Direct writing of tunable living inks for bioprocess intensification. U. S. patent filed October 5, 2018.
Baker, S., et al. 2018. Polymeric encapsulation of whole cells as bioreactors. U. S. patent filed October 30, 2018.