Christine Orme (17-LW-006)
Executive Summary
This research employs a novel combination of strategies for creating a new class of materials made from self-assembling nanocrystals in a colloidal solution for use in additive manufacturing. These customizable materials have application to nuclear stockpile stewardship and other national security applications.
Project Description
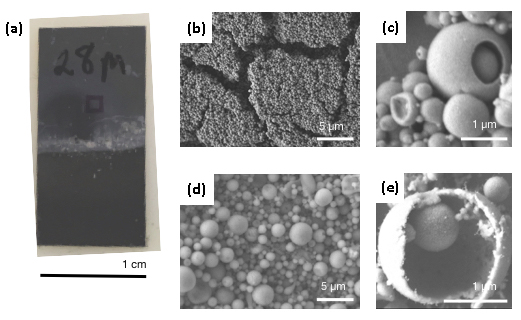
Nanometer-scale particles and crystals have become important building blocks for the discovery of materials with new functionalities. The next challenge is organizing these building blocks into useful intermediate-sized structures. Most research has focused on constructing super-lattices, which are dense materials composed of nanoparticles in a crystalline array. This research focuses on the opposite extreme―highly rarefied nanocrystal structures. By manipulating interfacial and adhesion energies, we intend to create a new class of porous materials, colloid capsule composite solids (selectively permeable capsules composed of nanocrystals), for use in additive manufacturing. To create these composites, we plan to couple two well-established synthesis strategies that have never been used in concert. We first make an emulsion in which nanocrystals self-assemble into these colloid capsules. If we then ensure the colloid composites have a slight net charge, they can be driven to a substrate surface by flowing the solution across an electric field, ultimately forming a film of nanocrystals organized into hollow spheres. The process is materials-agnostic, allowing metal, oxide, and organic solids to be used. It is also dynamic, allowing density and compositional variations to be controlled as the structure is grown. From these hollow particles we will create a cellular foam that can be expected to have excellent mechanical properties per mass once fused. This work is particularly compelling because Lawrence Livermore National Laboratory has developed a one-of-a-kind dynamic stereolithographic electrophoretic deposition system (akin to three-dimensional printing) that does not require patterned electrodes, and thus any three-dimensional pattern can be implemented by correlating the flow of materials and the optical pattern in a computer-controlled system. Diversifying the colloidal building blocks to shaped, hollow, or fluid-filled colloid capsules will have greater impact in a system capable of patterning in three dimensions.
As a result of this project, we expect to gain an understanding of colloid-capsule formation and stability such that three-dimensional structures can be engineered, creating new materials with densities lower than the best aerogels (less than 1 percent solid material by volume). We intend to couple two well-established synthesis strategies, Pickering emulsions and electrophoretic deposition, to create a new class of porous materials. This project will expand the materials set from simple colloids to complex, multi-component nanocrystal colloid capsules. We will perform controlled synthesis of single- and multi-component emulsions and films by shearing a discontinuous fluid in another continuous, immiscible phase that contains a stabilizing agent (e.g., surfactant, particles, or ions), and using sonication intensity (agitating particles with sound energy) to tune the shear rate. We will then quantify the emulsion kinetics and stability to control emulsion properties and deposition using dynamic light scattering, rheology, and imaging. These techniques provide information on zeta potential (stability of colloidal dispersion), mobility, viscosity, interfacial energies, and colloid-capsule size distributions as a function of solvents and nanocrystal properties (composition, concentration, and surface chemistry). Finally, we will demonstrate a multi-component catalyst system. Our three-dimensional porous structure would have a distinct advantage over current tandem catalysts (with two metal-oxide interfaces in close proximity for successive catalytic reactions) because of improved diffusion paths and higher access to interfaces.
Mission Relevance
This research supports the NNSA goal of advancing the science, technology, and engineering competencies that are the foundation of NNSA missions, namely stockpile stewardship. Specifically, the development of new building blocks that extend the Laboratory's current additive-manufacturing techniques to the ultra-low-density regime supports the advanced materials and manufacturing core competency. Furthermore, ultra-low-density metal structures that could be formed from this technology are required for high-energy-density targets used at the Laboratory's National Ignition Facility in support of stockpile stewardship, while porous materials with controlled pore shape and aspect ratio could be used to understand hot-spot generation in high-energy-density materials.
FY17 Accomplishments and Results
In FY17 we (1) set up a contract to perform emulsion-stability experiments and microtensiometer measurements with researchers at Carnegie Mellon University who have begun to alter their instrumentation and protocols to handle non-aqueous solvents; (2) successfully deposited metallic colloidosomes; and (3) began to improve yield, control size, and measure mechanical properties.