Timothy Rose (16-ERD-008)
Project Description
Post-detonation nuclear debris is the product of extreme chemical fractionation and mixing processes between the nuclear device and background material. These processes alter the chemistry of the nuclear debris so that it no longer reflects the chemistry of the source weapon. Radiochemical measurements of the debris must be corrected for these chemical distortions before the data can be used to draw inferences regarding the characteristics of the nuclear device. Empirical data correction methods that were developed during nuclear testing are still being used today. These methods are reliable, but offer no insight into the physical mechanisms controlling chemical fractionation. Our objective is to investigate chemical fractionation processes under conditions relevant to nuclear fireball condensation. This research will advance our experimental and theoretical knowledge of plasma condensation chemistry that is needed to build a science-based understanding of radiochemical correction methods. We will measure correlations between the physical and chemical properties of condensing plasmas at temperatures less than 1 eV using a steady-state inductively coupled plasma-flow reactor suitable for studying high-temperature chemistry in partially ionized plasmas. The flow rate of the system is adjustable, enabling the simulation of different cooling and mixing histories. This allows us to examine the interplay between cooling timescales and chemical fractionation patterns, and may offer insights into the relative roles of thermodynamic versus kinetic mechanisms in determining chemical partitioning. Comparison of the experimental results with data from historic nuclear tests will establish a critical link between the experimentally calibrated observables and the fractionation patterns in actual debris.
We will determine empirical fractionation ratios for pairs of chemical elements as a function of cooling and mixing rates, matrix composition, and oxygen fugacity (fugacity is the pressure needed at a given temperature to enable properties of a nonideal gas to satisfy the equation for an ideal gas). The sensitivity of chemical fractionation to these factors will be explored by varying each parameter over a range of values relevant to fireball condensation. Constraining uranium volatility is the top priority. This research will result in the first data set of its kind that directly relates fallout fractionation patterns to measured physical phenomena. The results will provide a basis for developing advanced computational models to simulate chemical fractionation using chemical kinetics or molecular dynamics codes. These models will, in turn, inform the development of predictive models for chemical fractionation patterns in fallout.
Mission Relevance
Developing a science-based experimental understanding of the correlation between fireball conditions and debris chemistry builds confidence in existing fractionation correction methods and may identify needed improvements, enhancing the Laboratory's strategic focus area in stockpile stewardship science. This work will leverage our broad knowledge of radiochemical distributions in debris, further supporting the core competency in nuclear, chemical, and isotopic science and technology.
FY16 Accomplishments and Results
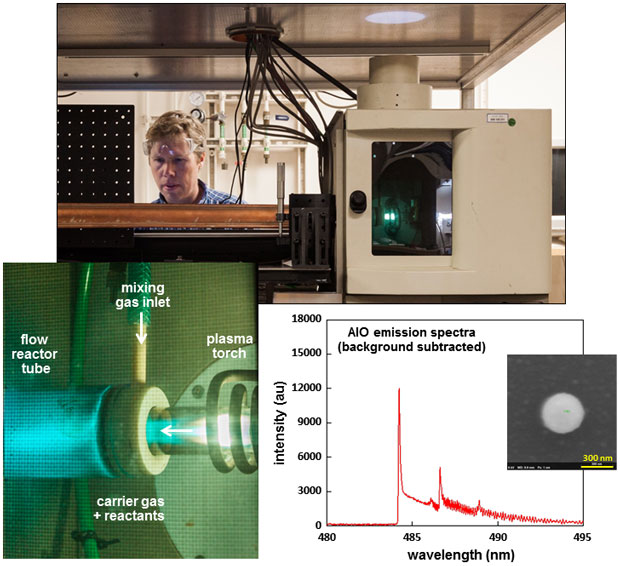
In FY16 we (1) developed and tested the flow reactor (see figure), (2) refurbished and tested diagnostic equipment, (3) developed an integration worksheet, (4) implemented safety requirements such as the reactor exhaust system, (5) collected physical data to refine our hydrodynamic model and particulate formation theory, and (6) experimented with the iron–oxygen system, making mid-infrared spectral measurements of molecular species for a range of oxygen conditions and initial characterization of particulates.
Publications and Presentations
- Koroglu, B., et al., Preliminary experimental results using a steady state ICP flow reactor to investigate condensation chemistry for nuclear forensics. 58th Ann. Mtg. APS Division of Plasma Physics, San Jose, CA, Oct. 31–Nov. 4, 2016. LLNL-ABS-697790.
- Rose, T., et al., Why nuclear forensics needs new plasma chemistry data. 58th Ann. Mtg. APS Division of Plasma Physics, San Jose, CA, Oct. 31–Nov. 4, 2016. LLNL-ABS-697038.