Alan Dehope (15-ERD-036)
Project Description
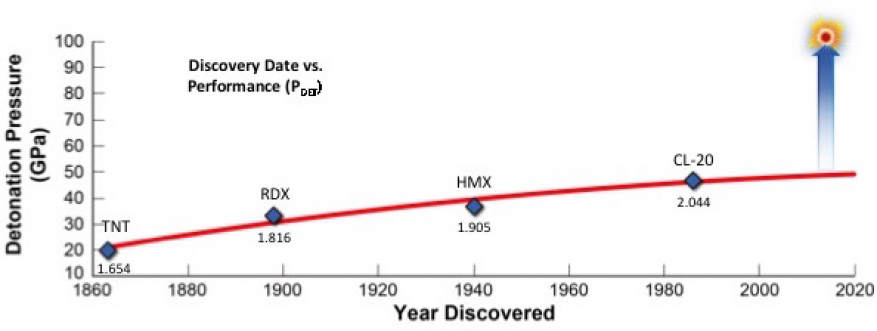
High explosives play an integral role in the nation's defense as components in conventional munitions as well as providing the energy source for nuclear weapons. However, the development of higher-performing explosives is limited by conventional organic-based explosives and their formulations. Significant improvement in explosive performance is an unmet scientific challenge that has persisted for decades (see figure). High-explosive performance is strongly dependent on material density. Therefore, we propose to explore whether we can realize large gains in detonation performance by exploiting the high densities and large amounts of stored chemical energy available in metal fuels. We intend to prepare energetic ligands (an ion or molecule that binds to a central metal atom) and high-power metal complexes to realize significant gains in detonation performance. We are proposing a path to increase detonation power by extracting the energy of metal oxidation (burning) in a detonation front. Such an increase in detonation pressure would have a groundbreaking effect on the field of energetic materials. These performance gains can be realized by leveraging recent organometallic chemistry methodologies that will allow for the creation of a new class of metallic explosives that possess fuel and oxidizer in the same molecule.
We expect to develop an oxidizer-rich organic donor-ligand molecule that we will form a complex with a low-valent metal fuel, forming a high-density energetic metal compound. The new material will contain metal fuel and oxidizer in the same molecule and will allow the oxidation to occur at a very fast rate, resulting in a detonation. The oxidation of the metal fuel will contribute to detonation pressure and velocity. Such a combination is a new concept in energetic materials research. Thermodynamic equilibrium calculations using the Cheetah code for modeling explosives predict significant improvements in detonation performance by burning metal in detonation. Such increases in material performance would enable the improvement of weapons systems performance, and create new applications for conventional weapons.
Mission Relevance
This research aligns well with the Laboratory's mission in national security, where advances in stockpile stewardship are needed. This work supports LLNL's core competency in advanced materials and manufacturing, which is relevant to the strategic focus area in stockpile stewardship science.
FY16 Accomplishments and Results
In FY16 we have (1) expanded our family of N-heterocyclic ligands containing multiple nitro groups on the aromatic rings, characterizing these ligands structurally using nuclear magnetic resonance spectroscopy—our work to date has not yielded air-stable metal complexes, precluding the characterization of the heats of formation and small-scale safety responses of these materials; (2) begun investigation of metal coordination reactions using model compounds that are safe to handle but are structurally very similar to our ligands of interest; and (3) developed multiple nitrated ligands sufficient for us to continue our metal coordination work.