David Weisz | 18-FS-035
Overview
Within the field of high-explosives (HE) science, the need to better understand HE chemistry drives research into interactions involving isotope substitution and other species of interest (e.g. tritium or uranium). This feasibility study was designed to investigate the potential of using laser ablation and optical emission spectroscopy to understand the high-temperature chemistry of an HE analogue with deuterium gas and, separately, with depleted uranium. The combination of laser ablation with optical emission spectroscopy is ideally suited for studying these interactions for two reasons: (1) emission spectra are highly sensitive to isotope substitution, making it easy to distinguish between chemical reactions involving 1 H or 2 H (deuterium), and (2) laser ablation emission spectroscopy is a rapid and cost-effective platform for studying the chemistry of HE.
Background and Research Objectives
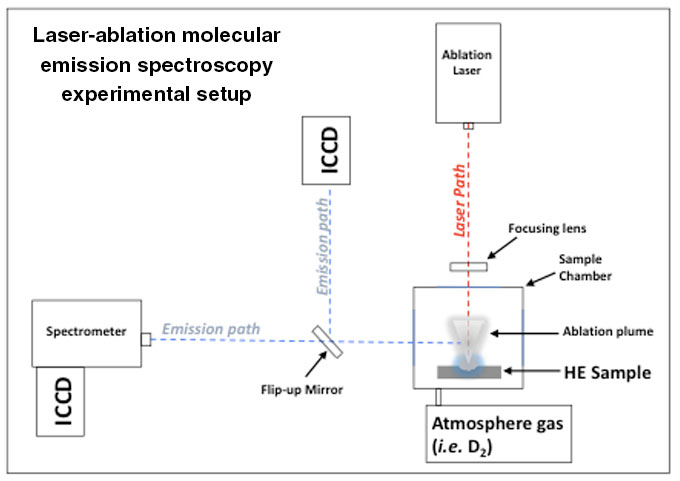
In order to accurately interpret the early chemistry of high-explosive detonations, scientists require an improved understanding of the chemistry between dissociated HE products and relevant species, such as tritium or uranium. One current topic of interest is in developing validated models of HE chemistry to understand how detonation products form. While tests using actual HE detonations provide model validation and valuable insight into these chemical processes, HE tests can often be technically challenging and expensive to execute. Laser ablation emission spectroscopy (see schematic below) provides a simple and cost-effective means by which to understand high-temperature chemistry and develop emission signatures relevant to actual HE detonations.
Simple schematic of the laser ablation molecular emission spectroscopy setup illustrating the ablation laser path as well as the emission path. The emission path can either be directed to a combination spectrometer and intensified charge coupled device (ICCD) for spectroscopy or simply imaged on the ICCD (not utilized for this study).
Laser ablation produces an initial plasma in excess of 10,000 K (~1 eV) (De Giacomo and Hermann 2017). In the condensing plasma, ions, atoms, and molecular species are initially in an excited state. The molecular species subsequently undergo rovibronic (rotational-vibrational-electronic) de-excitation. One can easily measure light from this de-excitation process using a dispersive grating and a detector to acquire characteristic spectra of these species (Bol'shakov et al. 2016, De Giacomo and Hermann 2017). Molecular emission spectroscopy has been shown to be effective for studying chemistry subsequent to the laser ablation of chemical compounds or pure elements reacting with surrounding materials(Harilal et al. 2016, Weisz et al. 2018, Serrano et al. 2015). It is also beginning to be applied to the chemistry of HE detonations (Glumac, N. 2005, 2017, Gottfried 2017, Kalam et al. 2017).
Prior to this study, we developed the laser ablation and emission spectroscopy technique to understand chemistry relevant to post-detonation nuclear forensics for the Defense Threat Reduction Agency (Finko et al. 2017, Koroglu et al. 2017, 2018, Weisz et al. 2017, 2018). This capability was used to observe the formation of a previously reported uranium oxide subsequent to laser ablation (Mao et al. 2017, Hartig et al. 2017), and we were able to confirm that this species was uranium monoxide (UO) by measuring the spectral shift in an 18 O and 16 O isotopic substitution study (Weisz et al. 2017). We have also used the laser ablation and emission spectroscopy technique to calibrate a simple kinetics model for oxide formation in a Sr, Zr, and O system, subsequent to the laser ablation of a SrZrO 3 target. Further, we were able to deduce the timescales of chemistry between oxygen from the sample and oxygen from the surrounding atmosphere, again using the spectral shift in an 18 O isotopic substitution experiment (Weisz et al. 2018). We are concurrently developing time-resolved IR spectroscopy and laser-induced fluorescence to track more complex molecular formation to continue applying the laser ablation technique to the study of post-detonation chemistry.
In order to understand the fundamental effects of low and high atomic number species (e.g. H and depleted U) on HE chemistry and relevant spectral interpretations, we proposed and accomplished the following research objectives:
Objective 1: To develop a signature of isotopic substitution with 2 H with HE-derived species (or a relevant surrogate) using laser ablation and emission spectroscopy in a deuterium-rich environment.
Objective 2: Develop a preliminary dataset illustrating the influence of uranium on the formation of diatomic HE-relevant intermediates by spectrally monitoring the formation of UO.
Impact on Mission
This study expands the field of HE science and supports the NNSA mission in stockpile stewardship.
Conclusion
Bol'shakov, A. A., et al. 2016. "Laser Ablation Molecular Isotopic Spectrometry (LAMIS): Current State of the Art." Journal of Analytical Atomic Spectrometry 31.1: 119–134.
De Giacomo, A. and J. Hermann. 2017. "Laser-Induced Plasma Emission: From Atomic to Molecular Spectra." Journal of Physics D: Applied Physics 50.18: 183002.
Finko, M. S., et al. 2017. "A Model of Early Formation of Uranium Molecular Oxides in Laser-Ablated Plasmas." Journal of Physics D: Applied Physics 50.48: 485201.
Glumac, N. 2005 "Aluminum Nitride Emission from a Laser-Induced Plasma in a Dispersed Aerosol." Journal of applied physics 98.5: 053301.
——— . 2013. "Early Time Spectroscopic Measurements During High-Explosive Detonation Breakout into Air." Shock Waves 23.2: 131–138.
Gottfried, J. L., 2017. "Laser-Shocked Energetic Materials with Metal Additives: Evaluation of Chemistry and Detonation Performance." Applied Optics 56.3: B47–B57.
Harilal, S. S., et al. 2016. "Shock Wave Mediated Plume Chemistry for Molecular Formation in Laser Ablation Plasmas." Analytical Chemistry 88.4: 2296–2302.
Hartig, K. C., et al. 2017. "Standoff Detection of Uranium and Its Isotopes by Femtosecond Filament Laser Ablation Molecular Isotopic Spectrometry." Scientific Reports 7.
Kalam, S. A., et al. 2017. "Correlation of Molecular, Atomic Emissions with Detonation Parameters in Femtosecond and Nanosecond LIBS Plasma of High Energy Materials." Journal of Analytical Atomic Spectrometry 32.8: 1535–1546.
Koroglu, B., et al. 2017. "Plasma Flow Reactor for Steady State Monitoring of Physical and Chemical Processes at High Temperatures." Review of Scientific Instruments 88.9: 093506.
——— . 2018. "Gas Phase Chemical Evolution of Uranium, Aluminum, and Iron Oxides" (unpublished manuscript).
Mao, X., et al. 2017. "Combination of Atomic Lines and Molecular Bands for Uranium Optical Isotopic Analysis in Laser Induced Plasma Spectrometry." Journal of Radioanalytical and Nuclear Chemistry 312.1: 121–131.
Serrano, J., et al. 2015. "Exploring the Formation Routes of Diatomic Hydrogenated Radicals Using Femtosecond Laser-Induced Breakdown Spectroscopy of Deuterated Molecular Solids." Journal of Analytical Atomic Spectrometry 30.11: 2343–2352.
Weisz, D. G., et al. 2017. "Formation of 238 U 16 O and 238 U 18 O Observed by Time-Resolved Emission Spectroscopy Subsequent to Laser Ablation." Applied Physics Letters 111.3: 034101.
——— . 2018. "The Effects of Plume Hydrodynamics and Oxidation on the Composition of a Condensing Laser-Induced Plasma." Journal of Physical Chemistry A 122.6: 1584–1591.
Publications and Presentations
Weisz, D., et al. 2018. "Isotope Substitution to Study High-Temperature Vapor-Phase Chemical Pathways." American Chemical Society National Meeting, Boston, MA, August 2018. LLNL-POST-756697.