John Despotopulos (17-LW-035)
Executive Summary
This basic science research is focused on expanding our understanding of the heavy metal flerovium and its placement in the periodic table. Both the knowledge gained and the tools developed in the process of the research will have application to nuclear forensic sample analysis, nuclear threat reduction, heavy metal remediation, and energy and resource security.
Project Description
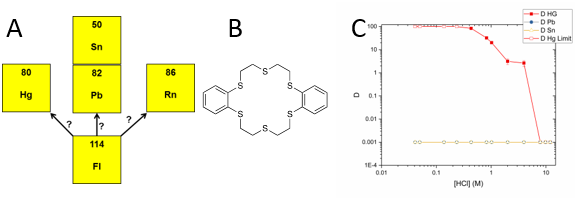
Over the past decade, superheavy element science has been resurgent with the discovery and naming of elements 113 through 118, and investigators are exploring the chemical properties of these transactinides to establish their placement within the periodic table. Because transactinides contain large numbers of protons, relativistic effects can cause electron orbital contraction, which alters the bonding characteristics and chemical behavior of transactinides compared to lighter members of the same chemical group (also known as homologs). Predictions for element 114, flerovium, indicate the potential for vast deviations in its chemical behavior compared to its homologs of lead, tin, and mercury. Currently there are no data on the aqueous-phase behavior of flerovium, and very few homolog studies have been reported. We are developing the first aqueous-phase chemistry of flerovium. Because flerovium has a very short half-life and low production rate, chemical methods must be rapid and easily automated. Researchers at Lawrence Livermore have previously shown that traditional oxygen-bonded ethers can extract flerovium homologs, but the extraction kinetics are too slow for a flerovium experiment. Sulfur-bonded thia-crown ethers, however, have increased affinity for mercury and lead. (Thia refers to the oxygen atom in a compound replaced by sulfur, and crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups.) We are synthesizing thia-crown ethers to produce isotopes of flerovium’s homologs at Livermore’s Center for Accelerator Mass Spectrometry (CAMS). We will then use these isotopes to develop a liquid-phase procedure for investigating the chemical properties of flerovium.
This project will result in a chemical separation method for the first aqueous-phase-based system for investigating flerovium. We expect to integrate synthesis of novel extraction ligands via known reaction pathways, establish methods for isotope production, and develop specific separation techniques. Our specific goals are to synthesize novel macrocyclic crown thia-ethers designed to improve the kinetics of extraction for the flerovium homologs with respect to previously investigated traditional crown ether analogs, as well as produce isotopes of the flerovium homolog elements lead, tin, and mercury via established methods at CAMS. We will use these isotopes to develop separation methods for identifying the chemical properties (i.e., the bonding coordination) of flerovium. Our work will also establish new ligands capable of sequestering specific elements, which has applications to remediation of heavy metals and sample analysis for nuclear forensics. Establishing the placement of heavy elements on the periodic table allows theorists to determine how nuclear and electronic forces interact. In addition, the chemical system developed through this work will be of interest to the DOE Basic Energy Sciences office, which has identified the importance of a new heavy-element research program designed to study the chemistry of transactinides. Additionally, our project will be useful in training the next generation of heavy-element radiochemists at Lawrence Livermore.
Mission Relevance
This project advances the NNSA goals of strengthening our science, technology, and engineering base and reducing nuclear threats. Development of novel separation chemistry is critical for maintaining Livermore’s expertise and leadership in radiochemistry and nuclear science and thus supports the Laboratory's nuclear, chemical, and isotopic core competencies. This competency is essential to the pursuit of several of the Laboratory’s research and development challenges, including nuclear threat reduction and energy and resource security. Novel ligand synthesis also has applications for heavy-metal remediation and forensic sample analysis.
FY17 Accomplishments and Results
During FY17, we (1) developed and used a novel technique to synthesize and investigate one of the proposed thia-crown molecules (dibenzo-hexathia-18-crown-6); (2) engaged in the investigation of a mercury-206 generator from purchased 210-lead; and (3) established a procedure that can yield mercury-206 for homolog studies without continual production runs at CAMS, because CAMS is still used to produce tin-113 for homolog studies, and performed some production runs.
Publications and Presentations
Despotopulos, J. D., et al. 2017. "Studies of Flerovium Homologs with Thiacrown Ethers." Presentation at the 9th Workshop on the Chemistry of the Heaviest Elements, Ascona, Switzerland, 8–11 October 2017. LLNL-ABS-730894.
———. 2017. "Studies of Flerovium Homologs with Thiacrown Ethers." Presentation at the 3rd International Symposium on Super-Heavy Elements, Kazimierz Dolny, Poland, 10–14 September 2017. LLNL-ABS-730894.