Monica Moya (14-ERD-005)
Abstract
The development of engineered tissue that represents human physiology will help increase the quality and predictability of therapies that move through the Food and Drug Administration approval pipeline and into clinical use. In vitro (outside the body) tissue models to date feature engineered organs that survive for only a few weeks and grow, at most, to a few hundred microns thick, primarily because of the difficulty of integrating vascular networks into artificially grown tissue. We addressed this challenge by using biological printing methods to assemble sophisticated capillary networks that can deliver nutrients through thick human tissue. Our four specific goals for this project included (1) development of printable biological ink, (2) coaxial printing and extrusion of a biological vessel, (3) vascular-membrane tissue characterization, and (4) perfusion studies for homogenous distribution of nutrients through multiple layers of thick tissue.
Background and Research Objectives
Only 10% of drugs entering human clinical trials are approved by the Food and Drug Administration for consumption,1 reflecting an overwhelming challenge in identifying effective therapeutics. Currently, animal testing is used as a proxy to assess organic and systemic level response to drugs in advance of human clinical trials. However, in many cases, animal testing does not accurately predict human safety and drug performance.2,3 Therefore, there is a need to develop engineered organs that better represent human physiology to increase the quality and predictability of therapies that move through the pipeline and into clinical care.
In vitro tissue models to date have featured engineered organs that survive for only a few weeks and grow, at most, to a few hundred microns thick. Based on our understanding of human physiology, a single monolayer of tissue is not representative of an entire organ. In most cases, achieving realistic function requires some degree of three-dimensionality, allowing cells to proliferate and communicate with neighboring cells in every direction.4,5 In addition, it has been shown that tissue found in vivo is only 200 microns from the closest capillary network.6,7 Beyond this distance, nutrients and oxygen diffusion is limited and tissue does not survive.8-10 Therefore, the primary reason that thicker tissue constructs have not been widely demonstrated is because of the difficulty in integrating vascular networks into artificially grown tissue.
Through internal R&D investments, the Laboratory has developed a bioprinting capability. This LDRD research focused on developing biological printing methods to assemble sophisticated capillary networks for delivering nutrients through human tissue. We have printed a functional 3D configuration of cells by optimizing the structural support, organization, and stability of the bio-ink and extracellular matrix. In addition to printing structures of defined shapes, our unique approach leverages cells’ self-assembly into their natural configuration.
Scientific Approach and Accomplishments
Optimized bioinks that encourage the formation of self-assembled vasculature
A key challenge in 3D bioprinting is developing and optimizing appropriate bioinks for the print process. Bioinks need to be optimized not only for the print process but also for long-term cell culture as this bioink becomes the scaffold with which cells will interact. Chemically, bioinks must have the right pH, the right ionic strength, and not be comprised of materials that could be toxic to cells. Physically, cells require a minimum amount of stiffness in their surrounding scaffold to build vasculature without the environment being so stiff as to stifle mass transport and proliferation.11 Often, 3D bioprinting studies have used synthetic polymers to aid in building a microfluidic network in a bioprinted platform.12 To continue heading in the direction of recreating more physiologically relevant and complex structures, it would be desirable to use organic materials, ultimately allowing cells to create their own vascular network and perfusable channels that more readily mimic human organs.
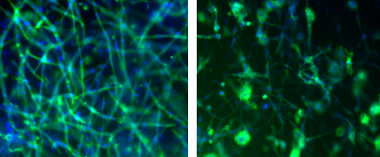
We have succeeded at developing fibrin-based inks that not only allow for a high-viability post-print process but also encourage vascular networks to form. Fibrin, a non-globular protein involved in the clotting of blood formed by combining fibrinogen and thrombin, is a common protein used in vascular development studies. Modifying a commercial off-the-shelf replicator printer, we have overcome the typical biomanufacturing challenges of cell damage and viability from mechanical stress during printing. Testing immediately after printing the cell-laden constructs showed viability of greater than 90%. Using high cell density (3 million cells/ml) bioinks, we 3D-printed geometries that were maintained in culture for 2 weeks. During this time, the metabolically active tissue constructs, consisting of co-printed human fibroblast and human umbilical endothelial cells, remodeled their surrounding scaffold and self-assembled into lumenized vascular networks with diameters ranging from 15–25 µm (Figure 1A). Our fibrin-based ink has also been shown to encourage vascular networks to form using human brain endothelial cell lines (Figure 1B). This result is very encouraging and shows the versatility of using our approach to create organ-specific vasculature.
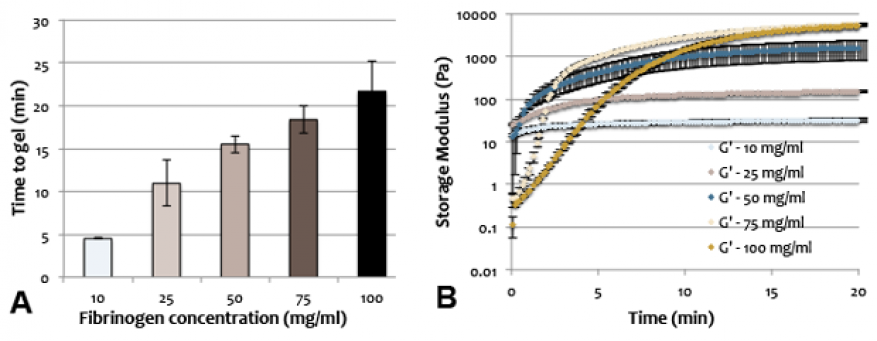
To specifically optimize the fibrin-ink for the print process and for the ultimate scaffold that it would become post-print, rheological measurements were done to quantify timing of gelation and final gel stiffness. Fibrin gels were characterized as a function of fibrinogen concentration, thrombin concentration, and gelation temperature. Gelation time was determined based on the approximate amount of time needed for G’ to reach steady state, indicating no further increase in stiffness due to the cross-linking (i.e., gelation) process. Steady state was defined as a consistent change of less than 1% between consecutive G’ measurements. Stiffness and gelation time were directly proportional to fibrinogen concentration. Changing thrombin concentration has a significant effect on the rate of gelation, but no effect on final stiffness. In terms of bioprinting, changing thrombin concentration would be imperative if the rate of gelation needs to be changed without affecting final gel stiffness and integrity (Figure 2A and B).
A base level of stiffness is needed for cells to perceive the scaffold as stiff enough to build onto, but if the gel scaffold is too stiff, cells will have limited mobility and there will be poor mass transport of nutrients.11 In addition to rheological studies, 3D static cell culture using bioinks was conducted to identify optimal final stiffness for vasculature formation. These results, combined with rheological measurements, yielded a basis by which other bioink candidate could be optimized. Understanding gelation rate coupled with gel stiffness allowed us to control the vascular density in the resulting 3D-printed construct. Using established protocols, a second bio-ink was optimized (fibrin-alginate-hyaluronic acid bioink) which also yielded high viability post-print (77%), which was both mechanically robust and conducive to cell vascularization.
Developed methods for coaxially printing vascular-like tubes for perfusing fluids using custom-made coaxial needles.
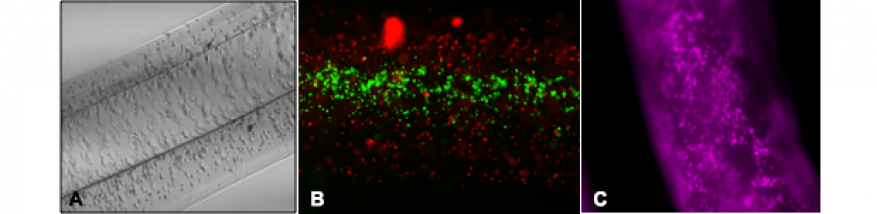
In addition to developing bioinks that could encourage self-assembly of vasculature, we also succeeded in creating bioinks that could allow for the 3D printing of defined geometries like tubular structures. While fibrin-based bioinks allowed for small vasculature formation, an alginate-based bioink was used to create larger vascular channels (500–800 µm) to allow for perfusion of media to feed the developing stroma (the supportive tissue) using the fibrin-based bioink. Using custom made co-axial needles (inside 24G, outside 16g), 5% cell-laden alginate gel was loaded into the outer needle while 1M CaCl2 was loaded into innermost needle. The two solutions were extruded using a dual syringe pump into a 100M CaCl2 bath. Extensive rheological studies were done to select the optimal concentration of UV-radiated alginate for printing. Robust patent alginate tubes were successfully printed. Tubes printed with stromal cells in the walls (Figure 3A) had high viability, 80–90%, as assessed by live/dead assay. High viability was still maintained after one week of culture.
To endothelialize the vascular alginate tube, various coatings and surface modifications were investigated. Although alginate has excellent biocompatibility, the alginate polymer itself is anionic (i.e., negatively charged) requiring surface modification for cells to attach. Various proteins coatings were investigated and a fibronectin/sodium citrate/calcium chloride mixture was selected to promote attachment. Cell-media mixture was perfused into the treated lumen of the vascular channels and allowed to sit overnight (Figure 3B). Human cerebral microvascular brain cells were found to attach well, but more importantly, using a CYQUANT proliferation assay, cells were found to proliferate well on the surface of the treated vascular tubes (Figure 3C). Attachment as well as proliferation is important for long-term culture under flow conditions.
Constructed a fluidic and housing device to connect printed tubes to external pumps to allow for perfused vascular networks
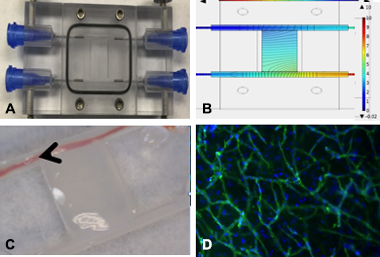
Key to the success of a bioprinted construct is survival past the initial print process. In vivo, cells are usually exposed to and responsive to both chemical and mechanical gradients. In order to develop our 3D-bioprinted construct in an environment with high physiological fidelity, we designed and constructed a bioreactor to dynamically culture our 3D-printed structures. Our custom reactor allowed for printed vascular tubing to be connected to external pumps and allowed the perfusion of media across fibrin-based printed stroma (Figure 4A and C). COMSOL, the multiphysics simulation code, was used to create a finite element model of the experimental design taking into account material properties such as permeability and porosity. The geometry of the bioreactor was meshed with approximately 800K tetrahedral elements. Brinkman equations (to govern slow flow through porous media) and the Navier–Stokes equation (to govern fast flow through the channels) was applied to model and compute for velocity and pressure (Figure 4B).
Cells in the bioreactor were cultured under static (Figure 4D) and flow conditions either as a dispersed co-culture or as a spheroid. Short-term (three-day) culture of endothelial and stromal cells cultured as spheroids showed biased sprouting from initial spheroid in the direction against the interstitial flow gradient, agreeing with what is seen in vivo. Randomly dispersed co-cultures of endothelial cells and fibroblast were successfully cultured for 12 days under flow conditions as confirmed by live/dead assay.
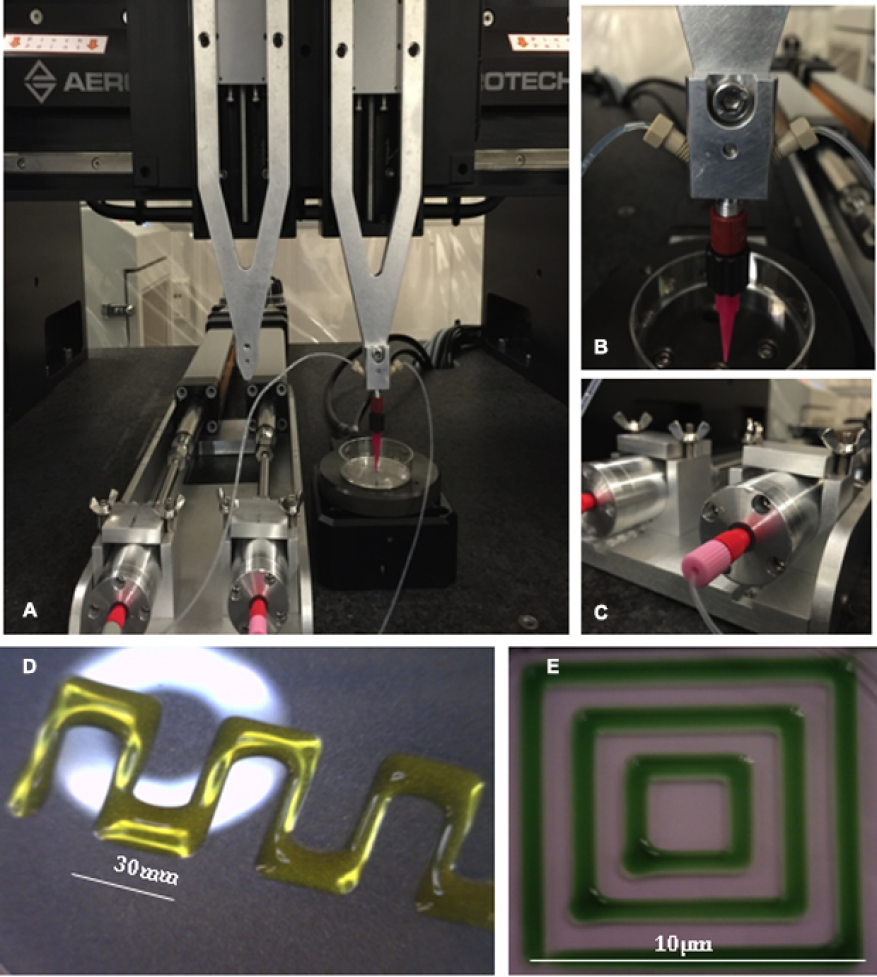
Integrated new Aerotech gantry motion control system for increased printing resolution with custom built displacement pumps for improved dispensing of gelling bioinks
Bioprinting specific geometries with live cells on the micron level requires precision, sub-micron electrical resolution, and repeatability. During this LDRD we designed a custom Aerotech gantry motion control system to enhance our bioprinting capability (Figure 5). Given the larger footprint compared to our older bioprinter, this system required construction of an enclosure to offer product and personal protection when printing with biologicals. A custom enclosure was also designed and constructed. We were able to successfully modify our optimized bioprinting protocols to work with the new gantry system and pumps with high viability. Endothelial and fibroblast cells bioprinted in fibrin gels using the Aerotech system were confirmed to be more than 77 percent viable after one day, and all bioprinted samples retained viability and sterility after one week of culture. To further increase our bioprinting capability, additional materials (without cells) for use with the new system were optimized with extensive rheological studies. These materials included pluronic-27, alginate-gelatin, and alginate-nanocellulose hydrogel.
Impact on Mission
Integrating complex microvascular networks in thick living tissue generates relevant tissue models that measure the therapeutic response to unknown chemical and biological agents, in support of a central Laboratory mission in national security in the area of biosecurity to develop platforms and tools for rapid medical countermeasures to emerging threats, which also supports the core competency in bioscience and bioengineering.
Conclusion
Our ability to implement advanced printing techniques to assemble biological material into sophisticated capillary networks will enable the creation of a sustainable platform of networks capable of distributing oxygen and nutrients homogenously through several layers of tissue, with the use of only natural biological materials. The goals of this project were achieved by developing a strong team of biologists and engineers at Livermore, as well as by forming strategic partnerships outside of the Laboratory. Our research developed capabilities in tissue engineering of interest to the Defense Advanced Research Projects Agency, Defense Threat Reduction Agency, National Institutes of Health, and Food and Drug Administration, all of whom have expressed interest in developing organ-on-a-chip technologies to accelerate research of host–pathogen, therapeutic, and vaccine interactions with human tissue.
References
- Kola, I., and J. Landis, “Can the pharmaceutical industry reduce attrition rates?” Nat. Rev. Drug Discov. 3, 711 (2004).
- Knight, A., “Systematic reviews of animal experiments demonstrate poor contributions toward human healthcare.” Rev. Recent Clin. Trials 3, 89 (2008).
- DiMasi, J. A., R. W. Hansen, and H. G. Grabowski, “The price of innovation: new estimates of drug development costs.” J. Health Econ. 22, 151 (2003). http://dx.doi.org/10.1016/S0167-6296(02)00126-1
- Griffith, L. G., and G. Naughton, “Tissue engineering—Current challenges and expanding opportunities.” Science 295, 1009 (2002). http://dx.doi.org/10.1126/science.1069210
- Gauvin, R., et al., “Application of microtechnologies for the vascularization of engineered tissues.” Vasc. Cell 3, 24 (2011). http://dx.doi.org/10.1186/2045-824X-3-24
- Haraguchi, Y., et al., “Development of a new assay system for evaluating the permeability of various substances through three-dimensional tissue.” Tissue Eng. C Meth. 16, 685 (2009). http://dx.doi.org/10.1089/ten.tec.2009.0459
- Muschler, G. F., C. Nakamoto, and L. G. Griffith, "Engineering principles of clinical cell-based tissue engineering", J. Bone Joint Surg. Am. 86-A(7), 1541 (2004).
- Yannas, I. V., Tissue and organ regeneration in adults. Springer, New York, NY (2001).
- Ko, H. C., B. K. Milthorpe, and C. D. McFarland, “Engineering thick tissues—The vascularisation problem.” Eur. Cell. Mater. 14, 1 (2007).
- Rouwkema, J., N. C. Rivron, and C. A. van Blitterswijk, “Vascularization in tissue engineering.” Trends Biotechnol. 26, 434 (2008). http://dx.doi.org/10.1016/j.tibtech.2008.04.009
- Sieminski, A. L., et al., “The stiffness of three-dimensional ionic self-assembling peptide gels affects the extent of capillary-like network formation.” Cell Biochem. Biophys. 49, 73 (2007).
- Wu, W., A. DeConinck, and J. A. Lewis, “Omnidirectional printing of 3D microvascular networks.” Adv. Mater. 23, H178 (2011). http://dx.doi.org/doi:10.1002/adma.201004625
Publications and Presentations
- Moya, M. L., and E. Wheeler, E., 3D Bioprinting Human Vasculature. (2016) LLNL-PRES-701001.
- Moya, M. L., M. Cardona, and E. Wheeler, 3D Bioprinted in vitro system of the microcirculation. (2015). LLNL-POST-670405.
- Moya, M. L., M. Cardona, and E. Wheeler, Bioprinting an in vitro system of the microcirculation. (2015). LLNL-PRES-674190.
- Moya, M. L., M. Cardona, and E. Wheeler, Bioprinting vascular networks for engineered tissue constructs. (2015). LLNL-PRES-676898.