Vanessa Tolosa (16-ERD-035)
Project Description
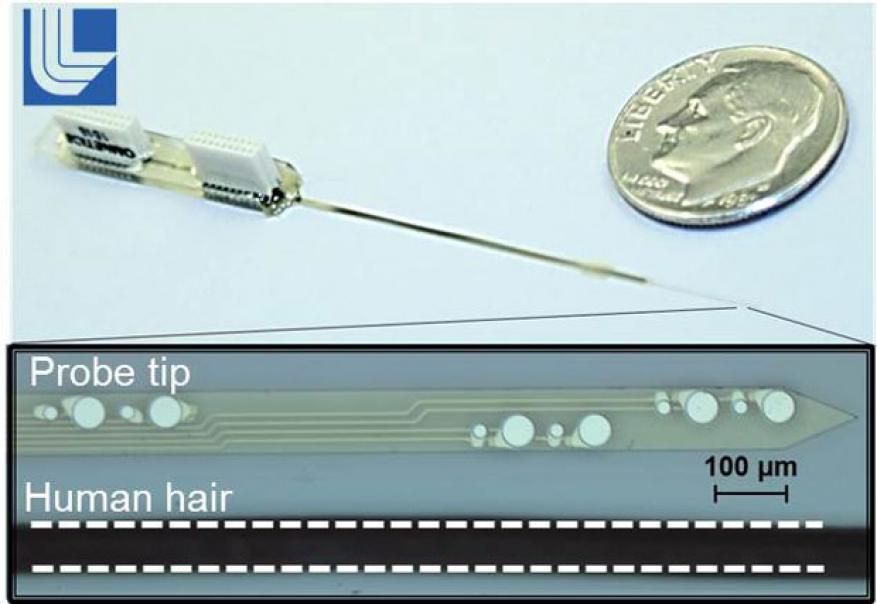
Biological systems communicate and function through a series of complex biochemical and electrical interactions. The ability to monitor these diverse interactions in real time and in physiologically relevant settings can be a powerful tool in determining the biological causes of diseases and uncovering the mechanisms underlying the body's response to chemical agents. There are abundant electrical recording instruments. However, only a fraction of the body's signaling mechanism is electrical—the majority is actually biochemical. Over 500 quadrillion chemical reactions occur in our bodies each day. Yet our understanding of the role of neurotransmitters, which are chemicals that transmit signals across synapses for relaying nerve impulses, is still primitive. This is evident by the high rate of relapse and tolerance to pharmaceutical drugs for neurological and neuropsychiatric diseases. We plan to enable a fundamental understanding of the body's response to bio-agents and drugs by developing an advanced chemical sensor platform (see figure). Individual sensors will monitor cell health by measuring multiple biomarkers simultaneously, in real time. Specifically, we will tackle the problems of sensor lifetime, sensor selectivity for new molecules, and sensor integration into useable platforms. To improve sensor lifetime, we will use a novel technique of chemically engineering enzymes with nanometer-scale complexes that improve enzyme stability and lifetime. To improve sensor selectivity, we will custom engineer strings of nucleotides to specifically recognize complementary strings of nucleotides on our target protein of interest. Lastly, we will enhance fabrication of current electrode arrays by incorporating carbon-based materials into a micro-fabricated array platform, thereby opening the possibilities for new sensor methods in vivo.
We are developing a chemical sensor platform that will enable scientific discoveries currently limited by technology. We will develop (1) enzyme nanometer-scale complexes to stabilize enzymatic sensors for chronic monitoring; (2) aptamers to detect protein-based biomarkers (aptamers are short deoxyribonucleic acid, ribonucleic acid, or peptide sequences selected from a pool of random sequences for the ability to bind to a target molecule of interest, similar to antibodies); and (3) a multielectrode-array carbon-based platform to detect multiple biomarkers. By accomplishing these tasks, we will expand the sensing portfolio of real-time electrochemical sensors. We will enable sensors to last longer, detect a wider variety of biomarkers, and perform on arrayed platforms allowing for simultaneous, multiple-analyte detection. The results will, for the first time, allow for the long-term study of the acetylcholine neurotransmitter system affected by nerve agents and open doors to sense protein-based biomarkers important to monitoring immune response. Our multielectrode platform will make nondestructive chemical sensing accessible to two- and three-dimensional biomolecular platforms that will be common place in the near future.
Mission Relevance
Our chemical sensors are directly applicable to a rapid, nondestructive method to monitor cell health that enables development of countermeasures against biological and chemical threats, in support of the Laboratory's strategic focus area in chemical and biological security. Furthermore, the sensors can be modified to allow for detection both in clinical settings and in the field. The core competencies in bioscience and bioengineering as well as advanced materials and manufacturing are critical for meeting the goals of this research project.
FY16 Accomplishments and Results
In FY16 we (1) formulated and tested chemically engineered nanometer-scale shells to stabilize enzymes to improve sensor lifetime, (2) initiated efforts to grow and pattern carbon-based electrode materials designed for chemical sensing, and (3) developed custom analytical systems that will allow for multi-modal sensing.