Michael Nielsen (16-FS-030)
Abstract
Liquid-cell transmission electron microscopy is a new characterization technique that could provide deep insights into a broad range of functional materials in real environments. Our goal was to determine the feasible functionality of a liquid-cell electrochemical transmission platform for an electron microscope by investigating the nanometer-scale evolution of a nickel-based electrochemical catalyst, using a system at Lawrence Berkeley National Laboratory's Molecular Foundry. A commercial electrochemistry stage for transmission electron microscopy was tested to determine its applicability for microscopes at Lawrence Livermore. Deposition of a nickel-based electrocatalyst was pursued as a material system for the purpose of testing the stage. The stage was found to be problematic with recurring issues in the electrical connections and vacuum sealing, which has thus far precluded a systematic investigation of the original material system. However, the electrochemical cells we obtained will enable continued testing. Furthermore, discussions with a second vendor, which released a similar electrochemical stage during the course of our study, have resulted in an upcoming long-term loan of their stage at Lawrence Berkeley for testing. In addition, low-loss electron energy-loss spectroscopy measurements on nickel-bearing electrolyte solutions led to a broader electron energy-loss spectroscopy investigation of solvents and salt solutions. These measurements form the basis of a manuscript in preparation on electron energy-loss spectroscopy measurements of the liquid phase.
Background and Research Objectives
Electrochemical liquid-cell transmission electron microscopy is a recent characterization technique with the potential to provide unique insights into a range of functional materials, including numerous programmatic efforts such as energy storage materials, accelerated corrosion, additive manufacturing, and biomimetic assembly. A commercial platform is available for in situ electrochemical experiments on the microscope, but its viability for quantitative measurements across a wide spectrum of materials is unknown. The goal of this study was to test the functionality of this tool through investigating the nanoscale evolution of a nickel-based electrocatalyst, in order to determine the value of acquiring a liquid-cell microscope holder compatible with the CM300 and Titan microscopes at Lawrence Livermore. This new capability would provide a characterization platform to directly image and collect structural and chemical information at the nanometer-scale of assembly mechanisms, phase transformations, and morphological changes in a variety of liquid-based systems under controlled conditions.
Scientific Approach and Accomplishments
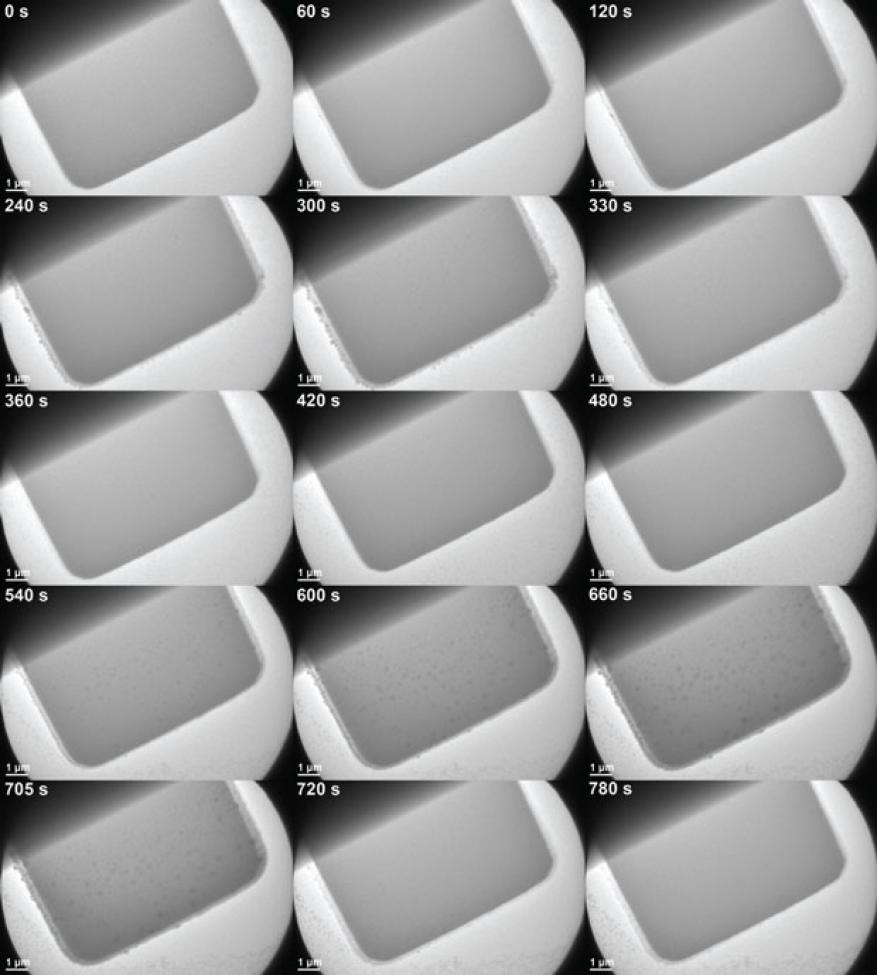
The primary research goals for this project were studying the deposition and evolution of a nickel-based electrocatalyst in the transmission electron microscope. To this aim, we tested an analytical electrochemical microscope stage by biasing electrodes immersed in a nickel-based electrolyte while imaging in a JEOL 2100F field-emission microscope at the Molecular Foundry. Initial biasing tests utilized a Metrohm PGSTAT302N potentiostat, which is an electronic instrument that controls the voltage difference between a working electrode and a reference electrode. However, because the potentiostat imposes a ground in the circuit and the stage goniometer holds the stage at 5 V, this setup was found to be incompatible. The experimental setup was thus changed to incorporate a Keithley source meter to manually sweep the relative bias of two electrodes in the electrochemical cell. We experienced recurring electrical issues with the microscope stage because of faulty and fragile electrical connections. Despite repeated repair of the delicate connectors, these problems have persisted, precluding the necessary ability to properly bias the electrochemical cell for testing. In addition, the stage exhibited issues with vacuum compatibility, which required it to be returned to the vendor for repair. In only a few instances did the electrical biasing aspect of the stage function as expected. In these cases we were able to cycle the bias on the electrodes and observe nickel deposition and stripping (Figures 1 and 2).
While the electrical issues in the stage prevented us from making extensive measurements on deposition and evolution of the catalyst material, we were able to capture the deposition and stripping of nickel from solution on a few occasions. A few cycles of this process, controlled by sweeping the electrode bias between +/−2.2 V, can be seen in Figures 1 and 2. In Figure 1, growth can be observed extending laterally from the edge of the electrode, in addition to islands nucleating and growing on the top surface of the electrode (most readily seen in the second cycle). However, deposits are also seen on the silicon nitride membranes separate from the electrode, starting around 360 s during the first dissolution sweep and building up throughout the rest of the video. Further work will need to be done to investigate whether this behavior is inherent to the cycling process or is a beam-induced phenomenon.
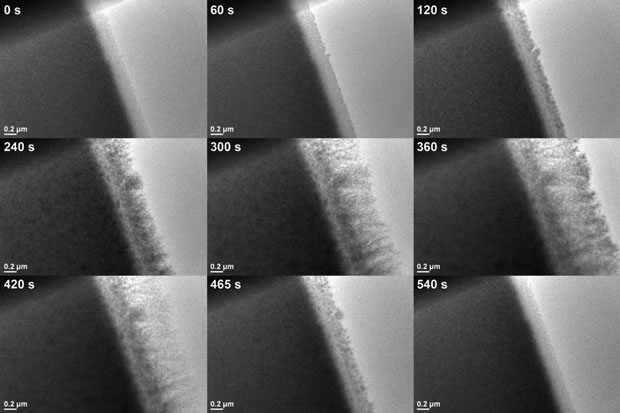
Figure 2 below shows a higher magnification view of the same cycling process, with just a section of the active electrode area in view. As with Figure 1, both lateral growth and islands on the top surface of the electrode can be seen growing and dissolving over time as the bias is again swept between +/−2.2 V.
Ancillary objectives included independent ex situ measurements of the stage and electrochemical cell performance. A measurement jig for the electrochemistry cell was designed and machined at Lawrence Berkeley, compatible with an existing probe station. However, because of problems with the stage detailed above, the importance of testing the cell independent of the stage has not yet been determined.
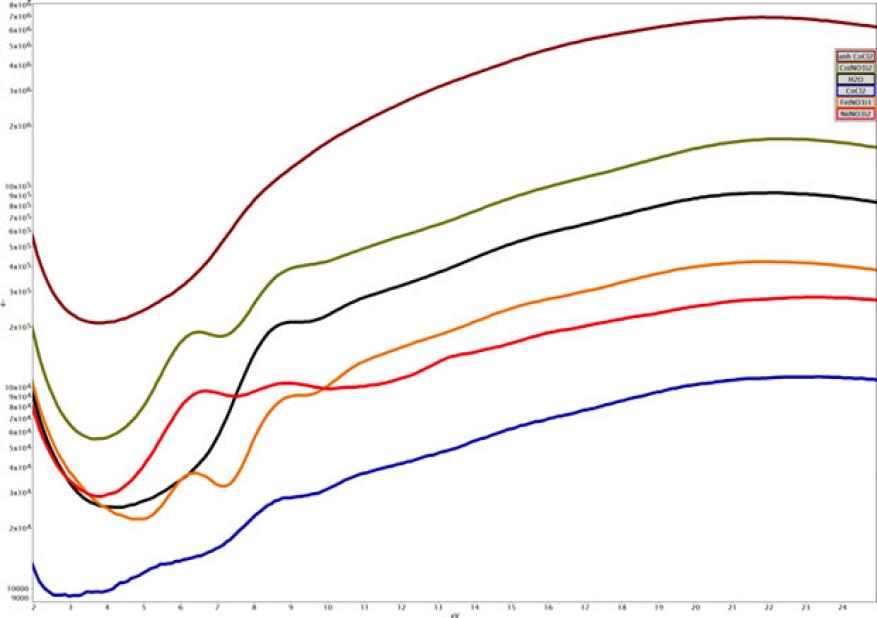
During the course of the electrochemical tests, electron energy-loss spectroscopy measurements were made of the deposited layer and nearby solution, in part to try to obtain a chemical signature of the material and its nearby environment. Very little exists in the literature on liquid-phase electron energy-loss spectroscopy signatures, beyond a handful of spectra of water and a few other solvents. In the nickel electrolyte solution used for electrodeposition, we found distinct low-loss features in the electron energy-loss spectrum that led us to look at a set of different salt solutions, as shown below in Figure 3. Analysis of this data is ongoing and we expect that it will form the basis of a manuscript on liquid-phase electron energy-loss measurements, a subject that is largely absent from the in situ transmission electron microscopy literature.
During the course of the project, a competing vendor released an updated model of their electrochemical transmission electron microscopy stage that addressed our previous concerns with their liquid stages, and we have arranged for the loan of a stage for testing at Lawrence Berkeley that is tentatively scheduled for early 2017.
Impact on Mission
Liquid-cell transmission electron microscopy is well aligned to current DOE Basic Energy Sciences efforts at Lawrence Livermore. The device's added capability would support Livermore's core competency in advanced materials and manufacturing. We investigated the utility of purchasing a commercially available electrochemical transmission electron microscope stage for the Laboratory, which would be compatible with the existing Titan and CM300 microscopes. Such electrochemical measurements would dramatically benefit programs investigating energy storage materials, accelerated corrosion, additive manufacturing, and biomimetic assembly. However, with the demonstrated lack of consistent functionality, the results suggest that it would not be feasible to purchase the stage at this time. However, the negotiated future loan of a competing stage for testing at Lawrence Berkeley may result in a suitable stage.
Conclusion
Recurring problems with the transmission electron microscope stage precluded any extensive investigations into deposition or aging of the nickel-based electrocatalyst. The current lack of consistent functionality under typical operating conditions makes the stage a poor candidate for use at Livermore. However, continued research of electron energy-loss using the remainder of the supplies purchased under this study may determine that the stage functions with further modifications. Additionally, the future long-term loan of a competing stage at Lawrence Berkeley for testing may determine that another vendor’s product is a viable solution. Electron energy-loss measurements on low-loss signatures from solvents and electrolyte solutions are currently being analyzed. Further measurements will be made with the aim to compile a manuscript for publication on quantitative chemical measurements of the liquid phase in transmission electron microscopy.