Sean Gilmore | 17-LW-051
Overview
Treating patients with highly invasive cancer remains a significant challenge in healthcare. In this project, we leveraged novel techniques in formulating small-molecule drugs and vaccine adjuvants using biomimetic nanoparticles (referred to as nanolipoprotein particles or NLPs ) to develop a combination chemotherapy-immunotherapy regime. This therapy was evaluated in vitro and in vivo using a model of highly metastatic breast cancer. In our treatment regime, which combines chemotherapy (doxorubicin) and immunotherapy, we observed a decreased tumor volume over the control group that was on par with free doxorubicin. We observed 100 percent survival in the group that received formulated doxorubicin with the adjuvant. The group that received the doxorubicin with the adjuvant included the most animals with fewer than 250 metastatic cells in the lungs, indicating that this was the most effective group at limiting metastatic invasion. All groups that received the immunotherapy treatment had one animal that did not have any detectable metastatic cells in their lungs. This data suggests that this chemotherapeutic-immunotherapeutic regimen has potential for producing better outcomes and enabling longer survival times in the treatment of metastatic cancer.
Background and Research Objectives
Chemotherapy is often unable to resolve cancer that has metastasized. In one clinical study, the median survival for women with metastatic breast cancer was approximately 13 months (Kassam et al. 2009). While the last several years have seen significant advances in the field of cancer immunotherapy, the most successful techniques have been limited to treatment of specific cancer types, such as B-cell lymphoma (Davila et al. 2014). Effective broad-spectrum treatments remain elusive. The principle challenge to cancer treatment is to enable an effective immune response from the host immune system. Chemotherapy can slow metastatic expansion, but ultimately immune cells must be recruited to eliminate cancerous cells (Emens and Middleton 2015) and surveil the host for re-emergence of these cells. The current hypothesis in the field of cancer immunotherapy states that the immune system is continually detecting and eliminating cells as they acquire a cancerous phenotype, and that cells able to evade or suppress immune activation are the ones that form tumors (Swann and Smyth 2007). In fact, the most successful immunotherapeutic treatments so far have involved “transplants” of effector T-cells that have been grown in vitro to recognize specific cancer markers (Perica et al. 2015).
An alternative approach is to activate endogenous effector cells against the tumor and promote proliferation of these cells. The main obstacle to this approach is the recruitment of other immune cell types by the cancer to inhibit activation of effector cells. These suppressive cells include regulatory T-cells, macrophage subtypes, and myeloid-derived suppressor cells (MDSCs) (Munn and Bronte 2016). These cells are found in and around the initial tumor, as well as at sites of metastasis where they may actually facilitate this process (Ouzounova et al. 2017). Thus, administration of immunostimulants alone has been shown to be insufficient to generate an anti-tumor immune response (Bodey et al. 2000).
To facilitate an anti-tumor immune response, we combined chemotherapeutic and immunotherapeutic techniques. We leveraged novel methods for formulating small-molecule drugs and vaccine adjuvants using a biocompatible nanoparticle platform, namely NLPs (Fischer et al. 2014), to develop a combination chemotherapy-immunotherapy regime. Specifically, our treatment regime combines doxorubicin (chemotherapy) and CpG dinucleotides (immunotherapy), and is designated as NLP-Doxorubicin/CpG.
In our treatment regime, a chemotherapeutic formulation is first administered intravenously to inhibit proliferation of metastatic cells, and to kill cells at the site of the main tumor. Tumor-cell death will temporarily relieve immunosuppression and provide a source of antigenic material that immune cells can process and recognize as abnormal (Emens and Middleton 2015). At a later time, an immunostimulant is administered directly to the site of the main tumor to promote immune activation against the tumor cells. Though the immunostimulant is given locally, activated immune cells should be able to spread throughout the host and attack metastatic sites and cells.
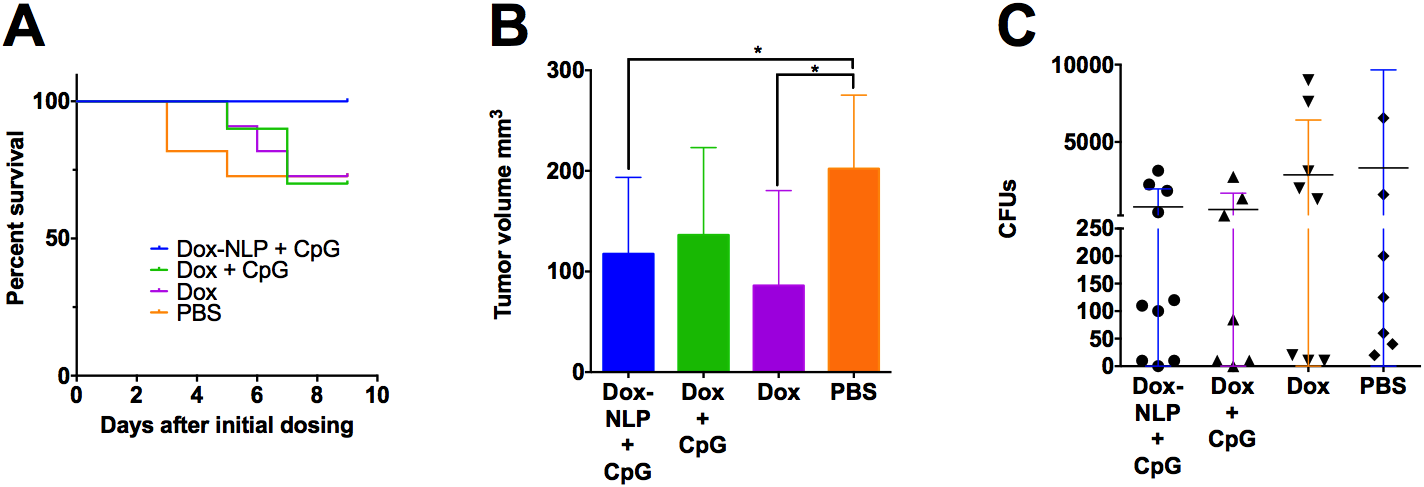
Over the course of a short (17 days) experiment, we observed complete survival of the subjects (mice) from the NLP group (see figure, part A). Furthermore, this group had the most animals (8 out of 10) displaying mild or no observable negative changes to their condition as a result of the cancer or drug treatment. Additionally, mice in the NLP-Dox/CpG group and the free-doxorubicin group had statistically smaller tumors than the phosphate buffered saline solution (PBS) control (see figure, part B). We did not observe any statistically significant differences among bacterial colony-forming unit (CFU) counts in the lungs as the distributions tended to be very bi-modal across all groups (see figure, part C). However, in all groups that received CpG, there was one animal that did not have any detectable metastases in the lungs. Taken together, this data indicates that administration of CpG is useful for managing metastases, but the chemotherapeutic drug is necessary to manage the growth of the main tumor.
A : Survival data from a 17-day experiment on mice treated with different therapeutic formulations. B : Tumor volume data collected at the end of the experiment. C : Metastatic 4T1 cells found in the lungs at the conclusion of the experiment. Results suggest that this chemotherapeutic-immunotherapeutic regimen has potential for producing better outcomes and enabling longer survival times in the treatment of metastatic cancer. Dox = Doxorubicin, NLP = nanolipoprotein particles, CpG = an immunotherapeutic, PBS = phosphate buffered saline solution, CFU = colony-forming unit.
Impact on Mission
Bioengineering on the nanometer-scale supports the NNSA goal of strengthening our science, technology, and engineering base. Specifically, this research develops the Laboratory’s core competency in bioscience and bioengineering by advancing the development of more successful cancer therapies by using a combination of chemotherapy and nanometer-scale, particle-based immunotherapy.
Conclusion
Our work demonstrated how chemotherapeutics can be combined with immunotherapy for improved treatment and investigated some of the mechanisms of immune response to cancer. We also investigated how small-molecule drugs can be packaged into nanoparticle formulations that exhibit performance superior to that of their free-drug counterparts. As a result of this project, we have gained a better understanding of how other small-molecule drugs could be incorporated into these particles and how they behave once administered. This knowledge has potential application in developing medical countermeasures using this particle platform.
Our primary goal at this time is to package the remaining data into a manuscript detailing our combined therapy. Additionally, we will be leveraging this data to apply for funding to continue this research through the Department of Defense’s breast cancer research program.
References
Alizadeh, D., et al. 2014. "Doxorubicin Eliminates Myeloid-Derived Suppressor Cells and Enhances the Efficacy of Adoptive T-Cell Transfer in Breast Cancer." Cancer Research 74(1): 104–118. doi: 10.1158/0008-5472.CAN-13-1545.
Bodey, B., et al. 1999. "Failure of Cancer Vaccines: The Significant Limitations of This Approach to Immunotherapy," Anticancer Research 20(4): 2665–2676. doi: 10.2217/nnm.14.16.
Davila, M. L., et al. 2014. "Efficacy and Toxicity Management of 19-28z CAR T-Cell Therapy in B-Cell Acute Lymphoblastic Leukemia." Science Translational Medicine 6(224): 224ra25. doi: 10.1126/scitranslmed.3008226.
Emens, L. A. and G. Middleton. 2015. "The Interplay of Immunotherapy and Chemotherapy: Harnessing Potential Synergies." Cancer Immunology Research 3(5): 436–443. doi: 10.1158/2326-6066.CIR-15-0064.
Fischer, N. O., et al. 2014. "Evaluation of Nanolipoprotein Particles (NLPs) as an In Vivo Delivery Platform." PLOS ONE 9(3): e93342. doi: 10.1371/journal.pone.0093342.
Kassam, F., et al. 2009. "Survival Outcomes for Patients with Metastatic Triple-Negative Breast Cancer: Implications for Clinical Practice and Trial Design." Clinical Breast Cancer 9(1): 29–33. doi: 10.3816/CBC.2009.n.005.
Munn, D. H. and V. Bronte. 2016. "Immune Suppressive Mechanisms in the Tumor Microenvironment." Current Opinion in Immunology 39: 1–6. doi: 10.1016/j.coi.2015.10.009.
Ouzounova, M., et al. 2017. “Monocytic and Granulocytic Myeloid Derived Suppressor Cells Differentially Regulate Spatiotemporal Tumor Plasticity During Metastatic Cascade.” Nature Communications 8: 14979. doi: 10.1038/ncomms14979.
Perica, K., et al. 2015. "Adoptive T-Cell Immunotherapy for Cancer." Rambam Maimonides Medical Journal 6(1): e00040e0004. doi: 10.5041/RMMJ.10179.
Swann, J. B. and M. Smyth. 2007. “Immune Surveillance of Tumors." Journal of Clinical Investigation 117(5): 1137–1146. doi: 10.1172/JCI31405.
Weilhammer, D. R., et al. 2013. "The Use of Nanolipoprotein Particles to Enhance the Immunostimulatory Properties of Innate Immune Agonists Against Lethal Influenza Challenge." Biomaterials 34(38): 10305–10318. doi: 10.2217/nnm.14.16.
Publications and Presentations
Gilmore, S. F., et al. 2018. "Dynamic Remodeling of Synthetic Lipoproteins in Blood Serum Solutions." American Chemical Society National Meeting, New Orleans, LA, March 2018. and LLNL-PRES-747343.