Elizabeth Wheeler (14-SI-001)
Abstract
The in vitro Chip-Based Human Investigational Platform (iCHIP) is an integrated, tissue-based assay system that models human physiological responses in order to accelerate the development of new drug entities and provide human-relevant data about mechanisms of both biological and chemical agent toxicity. iCHIP includes four tissue platforms. Because the peripheral nervous system is a target of many chemical agents, it was the first tissue system to be incorporated into the iCHIP platform. The central nervous system platform simultaneously cultures multiple neurons in a novel approach to recapitulating the human brain. The blood brain barrier platform incorporates flow and cultures cells in a 3D configuration. Finally, the cardiac platform enables simultaneous measurement of cell adhesion, health and contractility. All four systems have been validated by comparing our in vitro devices to known chemical stimuli responses in the literature from both in vitro and in vivo systems.
Background and Research Objectives
Development of new pharmaceuticals and antidotes to toxins currently takes years, costs billions of dollars, and relies extensively on animal testing. While animal testing is often used in the early stages of development, it can lead to inaccurate predictions about the human response to these chemicals and countermeasures. Animal models have proven inadequate to fully predict toxicity in humans. This failure to identify harmful effects is likely due to interspecies differences as well as key factors that are impossible to replicate in animals, such as the patient’s age, co-morbidities, and the simultaneous administration of multiple drugs. Ninety-two percent of therapeutics demonstrate serious unanticipated adverse events in clinical trials.
The iCHIP was developed to physiologically recreate the human body on a microfluidic chip. This low-cost alternative to human trials will ultimately allow scientists cheaper, quicker access to human-relevant data. While many other groups are working on various human tissue systems, we leveraged our previous experience with biocompatible electrode arrays, and focused on the following systems: peripheral nervous system, central nervous system, blood brain barrier, and cardiac. Each system resulted in a validated device. Systems were validated by confirming that the cells grown on the devices recapitulated human physiology. The behavior of cells after exposure to chemicals such as capsaicin, a component of chili peppers, or changes in their environments were compared to responses reported in the literature for either in vivo or in vitro systems.
Scientific Approach and Accomplishments
Peripheral Nervous System. The peripheral nervous system device leveraged our biocompatible electrode arrays and integrated fluid delivery to perform neurotoxic studies on human primary cells. The platform was designed to maximize cell health and longevity and incorporates: tightly controlled, live-monitored conditions in the cell growth chamber to optimize cell response; a microelectrode array for non-invasive electrophysiology recording; microscope compatibility to monitor cell health and response to chemical exposures; and microfluidics for automated fluid exchange. The surfaces of the cell growth chamber have been designed to support growth and adherence and have demonstrated long-term biocompatibility. The microelectrode array records cellular response without disturbing cell function, allowing for repeated recordings from the same neuron. Other assays performed on the platform include calcium homeostasis and immunocytochemistry.
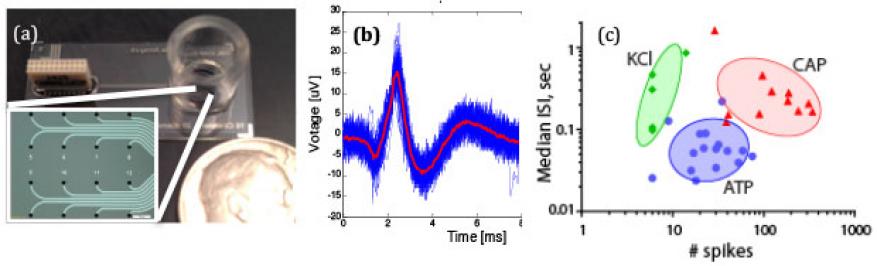
Human dorsal root ganglia were seeded on our device containing a microelectrode array (Figure 1a). The dorsal root ganglia were challenged with capsaicin (CAP), adenosine triphosphate (ATP), and potassium chloride (KCI), and the resulting action potentials were recorded using the microelectrode array (Figure 1b). Figure 1c shows a cluster plot of the dorsal ganglia response to these different stimuli. Key characteristics of the action potential such as spike interval and number of spikes were analyzed to validate that the dorsal root ganglia were responding in a physiologically predictive manner. Unlike patch clamping, the microelectrode array allows for long-term noninvasive monitoring of neuronal activity. This work represents the first long-term study of primary human diagnosis-related groups (DRGs) on a microelectrode array.
We also integrated biosensors into the iCHIP platform. In addition to being capable of electrical readings to determine the health of the neurons, the microelectrode array integrates pH sensors. The pH can be an indicator of cell activity, metabolism, and general cell health. Iridium oxide pH sensors have detected physiologically relevant pH changes on cells cultured for 15 days.
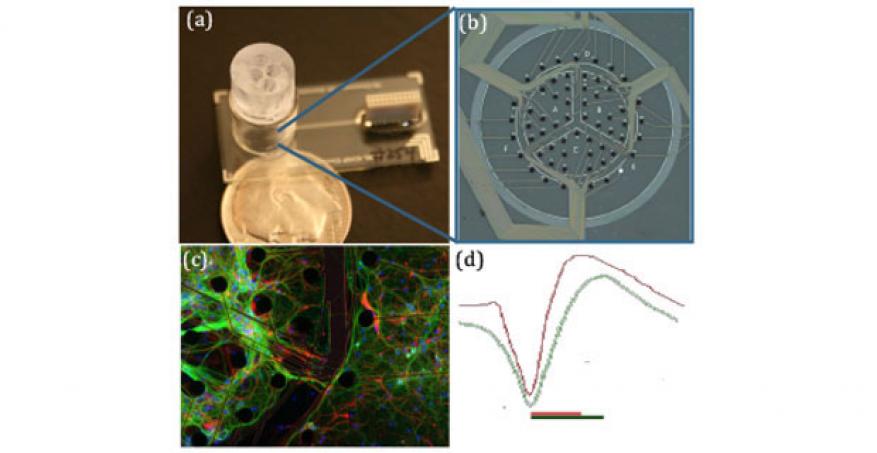
Central Nervous System. The central nervous system platform mimics the human brain by simultaneously culturing multiple brain sections on a single device without barriers or surface patterning between the sections of the brain. The individual brain sections (hippocampus, cortex, etc.) are initially seeded into different locations. Then, the processes from the neurons are able to extend and communicate with the other regions of the brain, creating the brain’s complex neuronal network. Depositing multiple neuron types within 100 microns required extensive development of a biocompatible microfabricated funnel, Figure 2a. Hippocampal and cortical neurons have been simultaneously cultured on the central nervous system platform and kept viable and physiologically relevant for longer than one month. The viability of the neurons has been confirmed both optically and electrophysiologically using an embedded microelectrode array, Figure 2b. As Figure 2c shows the neurons form complex networks across the white matter regions not initially seeded with neurons.
Central nervous system neurons were also infected with a Sindbis virus expressing green fluorescent protein. The infection dynamics and neuron response was measured in real time over the course of the infection using the microelectrode array. Neuron firing dramatically decreased after six hours post-infection, although obvious cytopathology was not detected.
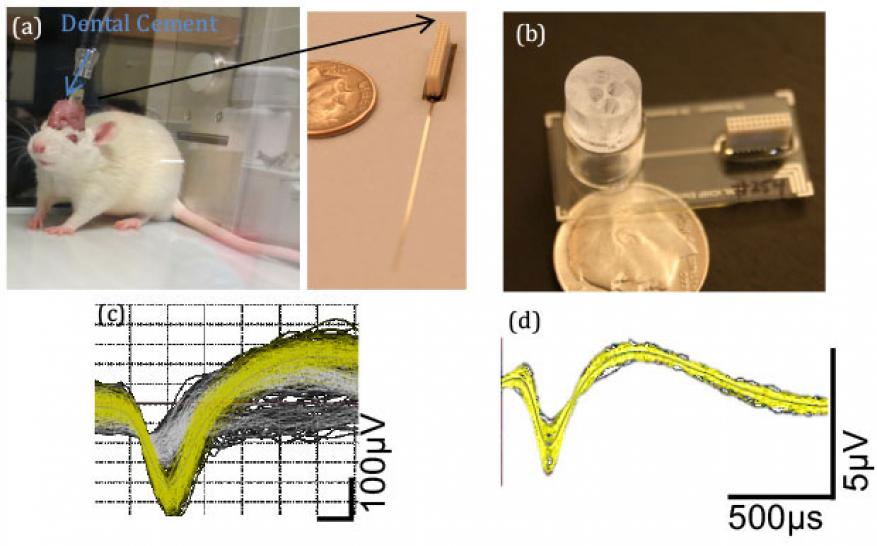
To fully validate the central nervous system platform, a direct comparison with in vivo results was performed in rodents. A flexible electrode array was implanted into the cortex of a rodent, Figure 3a. In parallel rodent hippocampal cells were cultured on the platform, Figure 3b. Representative electrophysiology recordings for both cases are shown in Figures 3c and d. In addition to recording baseline electrical activity, both in vivo and in vitro cases were exposed to an anesthetic dose of ketamine. As expected, both showed a repression of cortical neuron activity during the administration of the drug. However, the in vitro cells were more sensitive with a larger magnitude of decrease in cortical activity. These differences could be accounted for by changes in the microenvironment, increasing support cell composition and morphology (2d). Although not shown here, similar direct comparisons between in vivo and in vitro were performed for atropine (nerve agent treatment) and donepezil (treatment for Alzheimer’s). This work represents the most in-depth dataset to directly compare in vitro platforms with in vivo studies.
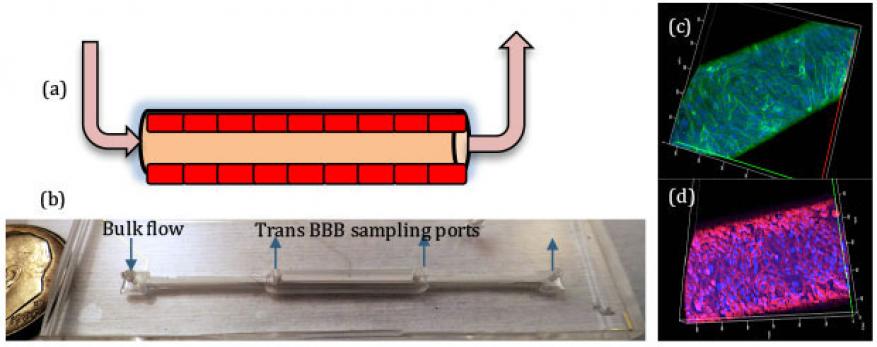
Blood Brain Barrier. The blood brain barrier serves a critical role in protecting the central nervous system by selectively limiting entry of compounds. Breakdown in the blood brain barrier is also involved in the pathology and toxicology in the brain. Our blood brain barrier incorporates a three-dimesional barrier with shear stresses to mimic the constant flow of blood in the human body. Endothelial cells are cultured on the inside of a porous hollow fiber until a tight uniform monolayer of cells is formed, Figure 4a and 4b. Figure 4c confirms that a confluent monolayer of cells (stained with CD31) has formed along the fiber. In addition to a confluent layer of cells, it is imperative that the cells form tight junctions to sufficiently control passage of chemicals. Figure 4d shows a confocal microscopy image of a representative blood brain barrier that has been stained with ZO-1 to highlight the necessary tight junctions. Flow is introduced via the outer ports at various time points in the culture.
The blood brain barrier device was validated by measuring the permeability of fluorescently labeled dextran, caffeine, and dopamine across the endothelial layer of cells. Compounds that cross the blood brain barrier were sampled via the inner ports and measured. As expected, caffeine is highly permeable and was detected at the trans blood brain barrier sampling ports. By contrast, dopamine has a low permeability and less was transported across the blood brain barrier. The cellular barrier is also responsive to chemical stimuli. Exposure to histamine is known to make the blood brain barrier leaky and tumor necrosis factor-alpha increases the blood brain barrier permeability. Both of these chemicals disrupted our blood brain barrier integrity and function via different mechanisms as expected.
Preliminary experiments seeding central nervous system neurons in a hydrogel in the subluminal area around the outside of the fiber allowed us to demonstrate the first integrated blood brain barrier and central nervous system platform. Although more experiments are needed, this preliminary data will be used in upcoming proposals to the National Institutes of Health.
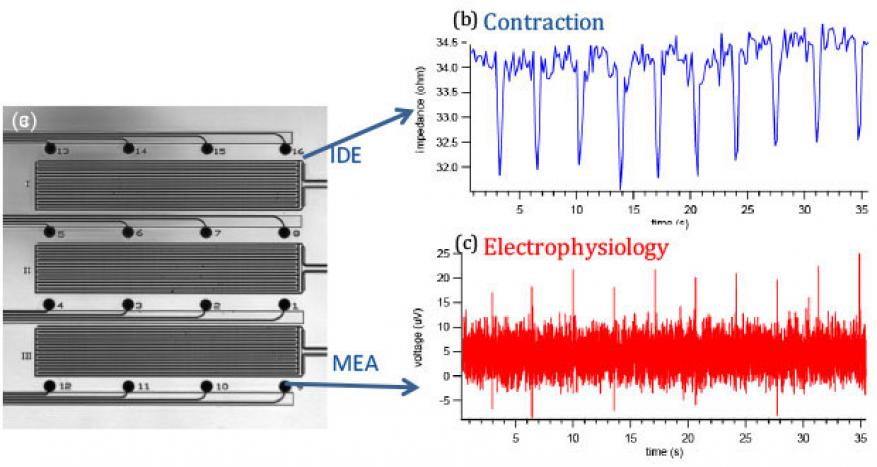
Cardiac. Our cardiac platform employs a novel design consisting of interpenetrating microelectrode and interdigitated electrode arrays that enable electrical stimulation, field potential mapping, contraction recording, and optical observation of cardiac cells and tissues. The platform integrates two independent yet interpenetrating sensor arrays for real-time noninvasive cardiac monitoring (Figure 5a). A microelectrode array similar to those used in the nervous systems enables field potential monitoring while an interdigitated electrode array measures impedance, which correlates with contraction. Cell adhesion and growth are measured using electrochemical impedance spectra, while high-speed impedance-time recording measures cardiac contraction. The underlying mechanism of monitoring contraction using an interdigitated electrode originates from the fact that the tissue layer functions as an insulator and current flow between the working and counter electrodes is impeded in direct correlation to the number of cells covering the electrode, the cell morphology, and the nature of the cell adherence. When cells contract, the gaps between the cells, cell layer and substrate are altered on the (sub)nanometer scale, leading to small changes in impedance. Working with collaborators at Harvard Medical School, Cambridge, MA, human induced pluripotent stem cell-derived cardiomyocytes were cultured as a model system.
Cardiac electrophysiology and contractility under physiological conditions and drug stimuli were measured, and representative measurements are shown in Figure 5b. The cardiac platform was validated by exposing the cardiomyocytes to norepinephrine and blebbistatin. Norepinephrine is a clinical drug used to treat low blood pressure and heart failure. As expected, the drug increased the tissue firing rate from 15 to 36 field potential spikes per minute, consistent with impedance data from the interdigitated electrode showing the same enhanced spontaneous beating frequency. However, the activation/propagation maps revealed a significant change in the propagation pattern and conduction velocity in presence of norepinephrine. This finding has been compared with computational efforts.
We further validated the platform by exposing the cardiomyocytes to blebbistatin, an excitation-contraction decoupling compound. Prior to blebbistatin exposure, the tissue beat spontaneously and rhythmically at a rate of 14 bpm. After incubation with 10 µM blebbistatin, the tissue was observed to be static under an optical microscope, and no contraction (impedance) was detectable, while the microelectrode array data showed the action potential pattern was not affected. Rinsing off the chemical restored the tissue contractility. The cardiac cells on our platform responded as expected when compared to human responses to blebbistatin. This platform provides a quantitative and predictive assay system that can potentially be used to comprehensively assess cardiac liability earlier in the drug discovery process.
Impact on Mission
Our development of the first in vitro platform for testing and characterizing toxins on neural and cardiac tissues supports the Laboratory bioscience and bioengineering core competency and addresses the national security mission in the area of medical countermeasures for rapid mitigation of evolving and unknown biological threats. Our research will enable timely medical countermeasures that treat disease or toxic effects from a terrorist attack that employs chemical or biological agents.
In addition, iCHIP has served as a pipeline to introduce young scientists to the exciting research at the intersection of engineering and biology. The project has attracted 8 postdoctoral researchers and 15 summer students.
Conclusion
In summary, we have designed and developed four validated human investigational platform systems: peripheral and central nervous systems, a blood brain barrier, and a heart model. Having validated each system, we now have the tools with which to begin addressing biomedical scientific questions and rapidly assess and predict the toxicity, safety, and efficacy of countermeasures against chemical and biological agents. Our research will reduce preclinical testing and improve relevance to clinical outcomes with technologies that utilize in vitro platforms with primary human cells organized in a physiologically relevant manner. The platform will also enable investigation of the mechanisms of infection for emerging threats, and it will be used to understand the evolution of threats in physiologically relevant human tissue. Human-on-chip systems that accurately reflect human physiology will enable the development of new therapies. They will also provide novel methods to investigate the impact of exposure to chemicals and toxins, and discover new biological mechanisms without relying on animal testing. Ultimately, predicting the impacts of potentially harmful chemicals, viruses, or drugs on human beings without resorting to live test subjects could transform the industry.
Publications and Presentations
- Enright, H. A., “Long-term non-invasive interrogation of human dorsal root ganglion neuronal cultures on an integrated microfluidic multielectrode array platform.” Analyst 141, 5346 (2016). LLNL-JRNL-674199. http://dx.doi.org/10.1039/c5an01728a
-
McNerney, M. W., et al., Calcium imaging of cultured DRGs exposed to an organophosphate pesticide. (2014). LLNL-POST-645723.
-
Soscia, D. A., Brain-on-a-chip: A device for advancing the understanding of the body’s most complex organ. (2015). LLNL-PRES-678890.
- Soscia, D. A., et al., Novel cell seeding funnel and microelectrode array (MEA) arrangement to separate and localize neurons from different brain regions. (2015). LLNL-POST-677732.
- Soscia, D. A., Overview of iCHIP CNS platform at LLNL. (2015). LLNL-PRES-675101.