Eric B. Duoss (17-FS-036)
Abstract
The purpose of this project was to demonstrate the feasibility of using three-dimensional (3D)-printed nanometer-scale porous metals for their various mechanical properties. Specifically, we developed two-ink 3D-printing systems based on gold and silver that were printed and post-processed to form an alloy. In this process, one of the alloyed phases was selectively removed (in a process called “dealloying”) to form a nanometer-scale porous metal. We demonstrated 3D printing of complex geometries with control over the alloy's features (with sizes ranging from 10 µm to >10 mm) and control over pore sizes (ranging from approximately 10 nm to 1 µm) via tuning of the post-processing methods. We also demonstrated the feasibility of using printed nanometer-scale porous gold for catalytic enhancement of reacting methanol to form methyl formate.
Background and Research Objectives
Creating materials with structural control over a wide range of sizes, from the nanoscale to the macroscale, continues to be a significant challenge. The ability to tailor structure across these dimensions would enable simultaneous programming of disparate functionalities (for example, catalytic reactivity, pressure drop across a reactor, and targets for laser-ignition research). Additive manufacturing (AM) and three-dimensional (3D) printing processes have gained widespread attention for their ability to rapidly and inexpensively create objects with excellent control over macro-, meso-, and sometimes microscale features (and in one instance, two-photon polymerization down to 100 nm). Lawrence Livermore National Laboratory has established itself as a leader in AM and 3D printing by envisioning and embracing a holistic approach to the AM process that addresses feedstock creation and production, modeling and simulation, optimized design, and invention of novel processes. In addition to AM, the Laboratory is also a longstanding leader in the development of nanometer-scale porous (i.e., “nanoporous”) materials, having developed numerous methods for manufacturing aerogels and nanoporous metals. For example, Livermore researchers have demonstrated the ability to create nanoporous gold with controlled microscale and nanoscale features using alloying and dealloying methods. While the Laboratory has demonstrated the application of these nanoporous metals to emerging areas such as energy storage and catalyst supports, it has proven difficult to program the macroscale and mesoscale structures for such materials. It is still difficult to additively manufacture objects composed of functional materials with microscale or nanoscale features while exercising control over both structure and composition.
The goal of this project was to demonstrate the feasibility of combining additive manufacturing with nanoporous metal production routes to obtain functional and hierarchical architectures with deterministically programmed structure (and composition) from the macroscale to the nanoscale. To our knowledge, our effort was the first demonstration of 3D printing of nanoporous metals, which represents a significant advance in the state-of-the-art.
Scientific Approach and Accomplishments
Monolithic nanoporous metals have a unique solid/void structure that provides both large surface area and high electrical conductivity for catalytic, sensing, energy, and optical applications. Many of these applications would greatly benefit from the integration of engineered macroporosity (i.e., pore sizes greater than 50 nm) to enhance and direct mass transport.
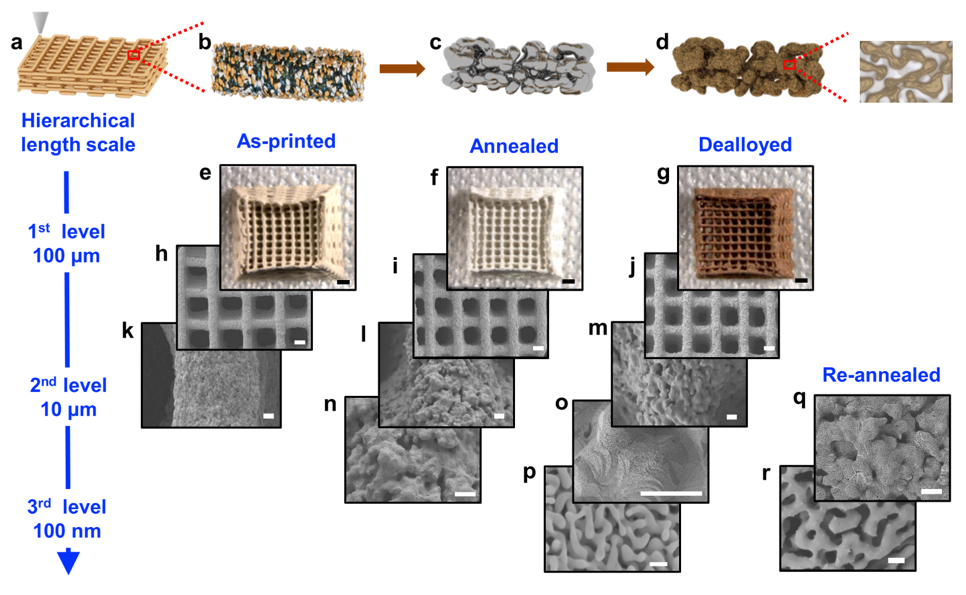
We have demonstrated the fabrication of hierarchical nanoporous gold (hnp-Au) with engineered architectures by combining 3D printing and dealloying (i.e., the selective leaching of a component of an alloy). The 3D-printed hnp-Au has three distinct pore/structural length scales: (1) the macro-architecture (10–1,000 µm), digitally programmed via 3D printing; (2) the microstructure (1–10 µm), controlled by organic solvent evaporation; and (3) the nanoscale structure, consisting of nanopores and ligaments introduced and controlled by dealloying and post-annealing (which fine-tunes the material’s physical properties, such as surface roughness). This universal approach can be applied to a variety of material systems.
Mass-transport performance tests revealed that the integrated engineered macroporosity accelerates electric-field-driven ion-mass transport by as much as one order of magnitude, and pressure-driven mass transport by as much as five orders of magnitude. Because of the latter, the gravimetric reaction rate of an hnp-Au catalyst membrane is twice as high as that of a non-hierarchical nanoporous gold catalyst membrane. The ability to dramatically accelerate and direct mass transport through architected hierarchical pores opens up a new design space for applications that require electrically conductive and catalytically active high-surface-area bulk materials, such as those used in flow batteries.
Bulk nanoporous metals that are manufactured by dealloying exhibit a unique morphology of interconnected nanopore channels and metallic ligaments, thus making them ideal materials for a variety of applications that require facile transport of both medium and electrons. This unique morphology is formed by self-organization processes during selective dissolution of the less-noble component of a binary starting alloy in a corrosive environment. For example, nanoporous gold (np-Au) can be fabricated by selectively dissolving silver (Ag) in Au–Ag alloys. Although the microstructure and feature size of these nanoporous metals can be controlled by the corrosion conditions (starting alloy, electrolyte, corrosion duration, potential, etc.), the dealloying process generally results in a unimodal pore-and-ligament size distribution. The resulting nanoporous metal exhibits a large surface-to-volume ratio, large open porosity (approximately 70 percent), and excellent electrical/thermal conductivity. However, slow diffusion-driven mass transport can significantly limit the performance of these metals in applications such as electrocatalysis, sensors, actuators, energy-storage devices, and materials separations. This limitation can be overcome by mimicking nature’s approach to this problem and introducing hierarchical cellular architectures, where macropores act as mass-transport “highways," while micropores (i.e., less than one micron in diameter) provide the high surface area required for functionality. Fast directional mass transport can be realized by incorporating anisotropic macropore architectures. The realization of engineered anisotropic architectures with multimodal pore-size distributions requires the development of new synthesis routes.
Two-step dealloying strategies involving additional annealing/re-dealloying steps have been used to generate np-Au membranes and bulk materials with bimodal pore-size distributions by selectively and sequentially removing Ag. Templating strategies have also been used to generate hnp-Au (for example, by coating sacrificial polymer templates with Ag–Au alloy films followed by template removal and dealloying). Although these methods have been successfully used to generate bulk hierarchical nanoporous metals, they are limited to a narrow range of materials. More importantly, they all lack the ability to generate deterministic, non-random, anisotropic engineered hierarchical network architectures that are needed to tailor mass transport and mechanical properties for specific applications such as microfluidic controllers, flow batteries, flow-through electrodes, and pressure sensors. To the best of our knowledge, engineered metal architectures with intrinsic hierarchical nanopores have not been realized.
Direct ink write (DIW)-printed hnp-Au (DIW-hnp-Au) samples with engineered architectures and multiscale hierarchical pores were fabricated through a sequence of printing, annealing, and dealloying steps (Figure 1).
We first developed a gel-based viscoelastic Ag–Au 3D-printable ink by mixing Au and Ag clays at the desired atomic ratio (Ag:Au = 70:30). This ink displays both a shear-thinning behavior to facilitate flow under pressure and a rapid pseudo-plastic-to-dilatant recovery time after deposition to enable shape retention. This composite ink can be extruded through a micro-nozzle onto a substrate mounted on a computer-controlled motion stage to yield the desired 3D sample shape and macroscale architecture. Before removing the printed "green" parts from the substrate for further processing, they were air-dried for several hours. At this stage, the printed structure consists of discrete Ag and Au particles that are glued together by a polymeric binder (Figure 1b). To form a homogeneous Ag–Au alloy, printed structures were annealed for 12 hours at 850°C in air (Figure 1c). Thermal decomposition of the polymeric binder leads to the formation of a microporous network structure in the printed filaments. Finally, nanoscale pores are generated by immersing the part in concentrated nitric acid (HNO3) to selectively dissolve Ag (Figure 1d). The DIW approach described herein provides digital control of the sample shape and the 3D macroscale architecture with excellent structural integrity and micro-architecture accuracy.
The DIW-dealloying strategy results in a hierarchical structure with three distinct length scales. The first level structure, digitally controlled by the 3D-printing process, is defined by 150-µm-wide filaments with a spacing of approximately 300 µm. The observed color change from the dark-grey color of the as-printed sample to silver and dark golden brown for the annealed and dealloyed samples, respectively (Figure 1e–g), reflects the compositional changes during annealing (water/organic binder removal and Ag–Au alloying) and dealloying (selective dissolution of Ag). The shrinkage of the sample during sintering (i.e., compacting and forming a solid mass of material by heat or pressure) is isotropic and depends on the solid loading of the ink. Typically, a volume shrinkage of less than 10 percent is observed during sintering; shrinkage during dealloying is negligible. The related changes in the microstructure are revealed by the SEM micrographs shown in Figure 1h– j. The as-printed filaments display discrete, densely packed Ag and Au particles with only small voids between them (Figure 1k). The second level of structure (consisting of approximately 10-µm voids) is caused by removal of the organic binder and water from the ink during thermal annealing of the as-printed structure. Simultaneously, large facetted grains form due to Ag/Au particle coarsening and Ag–Au alloy formation (Figure 1l/n and m/o). Control experiments show that second-level pore size and porosity can be tuned by varying the binder concentration in the ink. The third level of structural hierarchy of DIW-hnp-Au with approximately 30-nm-wide ligaments and pores is formed by dealloying (Figure 1p). The cross-sectional view of a fractured filament confirms the uniformity of the nanoporous structure throughout the dealloyed filaments. Energy-dispersive spectroscopic analysis of DIW-hnp-Au reveals approximately two percent of residual Ag, which is like the residual Ag content of bulk np-Au obtained through free corrosion. Adding a second annealing step after dealloying enables further tuning of the feature size of the third-level porosity of DIW-hnp-Au. Figure 1q and Figure 1r depict an example of a DIW-hnp-Au sample whose third-level feature size increased from 30 to 500 nm by re-annealing at 500 °C for one hour. It is important to note that the second annealing step only affects the third-level feature size because the temperature and time are not high enough and long enough to coarsen the larger-scale structures.
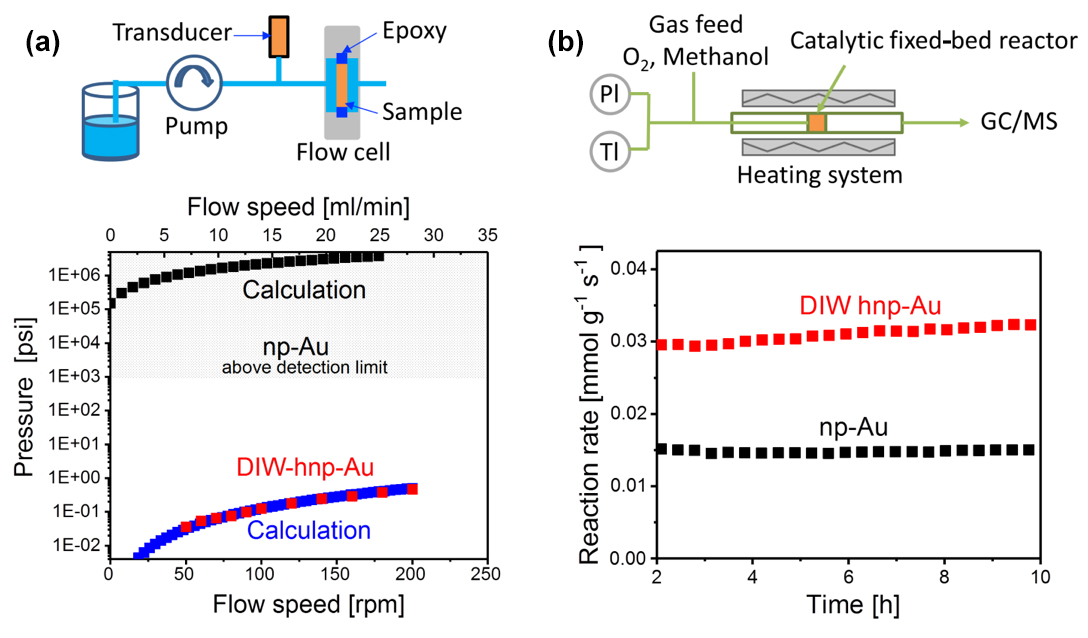
DIW-hnp-Au also shows dramatically improved pressure-gradient-driven mass-transport properties in both the liquid and gas phases (Figure 2).
Pressure-gradient-driven liquid-phase mass transport was evaluated by using a flow-through cell setup and comparing the pressure drop across both np-Au and DIW-hnp-Au membranes as a function of the liquid flow rate. The measured pressure-drop data of both membrane materials were then compared with calculations based on fiber beds for np-Au and a square-tube model for DIW-hnp-Au (Figure 2a). For np-Au, the pressure drop for all flow rates exceeded the upper range limit of the pressure sensor (15 psi), which is consistent with the calculations that indicate a pressure drop across the np-Au membrane in excess of 105 psi. For DIW-hnp-Au, the pressure drop is less than one psi under all experimental conditions. The total pressure drop in a single tube of the square lattice may be caused by the viscous pressure drop associated with flow down the tube and the pressure loss associated with discharge from the small orifice at the tube exit. The expected laminar pressure drop in this system is orders-of-magnitude smaller than the already small experimentally observed pressure drop. Moreover, we do not see the expected linear scaling of pressure drop with flow rate that would be expected if viscous affects dominated the system. Instead, the pressure drop-flow rate dependence suggests the presence of a pressure-drop mechanism controlled by jetting through a small orifice at the tube exit, as calculated by computational fluid dynamics and closely approximated by application of Bernoulli’s equation. The calculated results are in good agreement with the experimental data.
The pressure-gradient-driven mass-transport properties also directly affect the performance of np-Au-based membrane catalysts, as demonstrated by the example of the selective partial oxidation of alcohols (Figure 2b). Np-Au catalysts display excellent long-term stability compared to supported Au nanoparticle-based catalysts that often show rapid loss of reactivity due to particle agglomeration; however, optimization of mass-transport properties in these bulk catalysts remains a major challenge. When tested in a flow reactor, np-Au and DIW-hnp-Au membrane catalysts showed similarly high selectivity (70–90%) toward the formation of methyl formate, thus confirming that the DIW-hnp-Au and np-Au catalysts have very similar Ag–Au surface compositions. The reaction rate per catalyst weight, however, is two times higher for the DIW-hnp-Au sample compared to that of np-Au (Figure 2b). The more efficient use of the catalytic sites in DIW-hnp-Au directly reflects the improved mass transport of DIW-hnp-Au.
Impact on Mission
We demonstrated the feasibility of 3D-printing nanoporous metals using an alloying–dealloying method for the first time. These materials have novel structural, mechanical, and functional properties with applications in energy security, such as advanced reactors that are designed for catalytic behavior and pressure drop, as well as carbon dioxide reduction. This study also supports the Laboratory’s core competencies in advanced manufacturing and structural materials.
Conclusion
We have demonstrated the fabrication of hnp-Au microlattices with engineered shape and macroscale morphologies with three levels of porosities using a combination of 3D-printing techniques and dealloying. Our approach is simple and straightforward, and facilitates control of a macroscopic sample's shape without the need for machining, thus facilitating integration of these materials into functional devices and applications. We believe that optimization of mass flow through engineering of the 3D pore morphology will open the door to new high-power density flow-through technologies, including flow batteries and electrochemical CO2 reduction for daily and seasonal power-grid stabilization.
Publications and Presentations
Z. Qi, et al. 2017. Hierarchical porous metals with deterministic 3D morphology and shape via dealloying of 3D printed alloys. US patent application #15/790810, filed Oct. 23, 2017.