Elizabeth Wheeler (14-SI-001)
Abstract
Building upon a developmental platform for investigating primary human dorsal root ganglion cells, we propose to integrate human organ systems into an instrumented, micro-fluidic platform. Our ultimate objective is to create a highly integrated, multiple-organ, human-relevant in vitro platform to reproduce in vivo physiological response. We intend to develop tissue systems including dorsal root ganglia, central nervous system neurons, the blood–brain barrier, and cardiac tissue. We envision that this platform can be used to rapidly assess and predict the toxicity, safety, and efficacy of countermeasures against chemical and biological agents. Our research will reduce preclinical testing and improve relevance to clinical outcomes with technologies that utilize in vitro platforms with primary human cells organized in a physiologically relevant manner. The platform will also enable investigation of the mechanisms of infection for emerging threats, and it will be used to understand the evolution of threats in physiologically relevant human tissue.
We expect to deliver a validated human investigational platform with four human tissue systems of dorsal root ganglia, central nervous system neurons, blood–brain barrier, and cardiac tissue. We will validate this platform against known chemical agents to determine its performance compared to in vivo toxicity data. Our five main milestones are (1) determine protocols and techniques to harvest, digest, and culture tissue systems; (2) determine the appropriate assays to quantify cell viability and response to exposures; (3) develop the platform with integrated fluidics and electrodes; (4) demonstrate the in vitro platform with tissue systems; and (5) validate the in vitro platform with known chemical agents.
Mission Relevance
Our development of the first in vitro platform for testing and characterizing toxins on neural and cardiac tissues supports the Laboratory bioscience and bioengineering core competency and addresses the national security mission in the area of medical countermeasures for rapid mitigation of evolving and unknown biological threats. Our research will enable timely medical countermeasures that treat disease or toxic effects from a terrorist attack that employs chemical or biological agents.
FY15 Accomplishments and Results
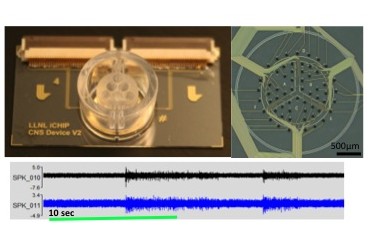
In FY15 we (1) exposed neurons on the peripheral nervous system platform to chemical agents at Livermore's Forensic Science Center; (2) conceived and fabricated a central nervous system platform for our in vitro device designed to localize cells from up to four different brain regions into designated areas on a micro-electrode array (see figure); (3) performed cell recording experiments, including studies of how cells from different brain regions, when allowed to communicate within their own population and with other regions, are affected by exposure to chemicals of interest; and (4) demonstrated integrated pH sensors on an in vitro platform for the peripheral nervous system and detected physiologically relevant pH changes in the presence of cells cultured for 15 days.
Publications and Presentations
- Enright, H. A., et al., Long-term non-invasive interrogation of human dorsal root ganglion neuronal cultures on an integrated microfluidic multielectrode array platform. (2015). LLNL-JRNL-674199.
- Soscia, D. A., Brain-on-a-chip: A device for advancing the understanding of the body’s most complex organ. (2015). LLNL-PRES-678890.
- Soscia, D. A., et al., Novel cell seeding funnel and microelectrode array (MEA) arrangement to separate and localize neurons from different brain regions. (2015). LLNL-POST-677732.
- Soscia, D. A., Overview of iCHIP CNS platform at LLNL. (2015). LLNL-PRES-675101.