Carlos Valdez (14-ERD-048)
Abstract
New technologies for capturing and catalytically degrading chemical weapon agents represent a critical national security need. This project addressed the use of molecular-complex scaffolds known as cyclodextrins that possess chemical and physical characteristics suitable for detection and analysis, decontamination, and medical countermeasures where the demand for broad-spectrum solutions is urgent. The project's dual components allowed us to obtain results with the overall goal of capturing and degrading chemical warfare agents. The first component focused on the synthesis and evaluation of zinc-based amino complexes that can be used as catalytic and stoichiometric entities for the destruction of organophosphorus-based compounds—that is, pesticides and nerve agents. The second component involved using carbohydrate-based scaffolds known as cyclodextrins as hosts for the capture and in some instances the degradation of toxic substances of interest in the chemical warfare arena as well as illicit, highly toxic substances such as fentanyls, which are potent, synthetic opioid pain medications.
Background and Research Objectives
An urgent need exists for the development of technologies directly aimed at high-priority chemical weapon agents in three areas: decontamination protocols, specificity of detection and analysis, and medical countermeasures. Current strategies to mitigate the environmental and physiological effects of exposure to chemical weapon agents have included complex biological solutions for patient care and stoichiometric, small-molecule approaches to surface decontamination. Our plan was to develop and validate an integrated experimental and computational approach for developing cyclodextrin-based nanometer-scale scaffolds for the capture and catalytic destruction of organophosphorus nerve agents and fentanyls. Successful technologies have been developed to mitigate individual issues for known chemical threats. However, establishing broadly applicable and rapidly responsive methodologies, targeted at known and more importantly emerging threats, remains critical. Although our research addressed the development of designer cyclodextrin molecules for two classes of agents, the foundation of our work was an integrated experimental and computational strategy for the discovery of efficacious cyclodextrins for broad-spectrum application.
Our project explored the use of cyclodextrins, which are supramolecular scaffolds possessing chemical and physical characteristics uniquely suitable for demonstrating the efficacy of this capability in the three areas described above. Our research focused on two classes of chemical warfare agents: the organophosphorus-based nerve agents and a series of incapacitating agents known as fentanyls. Both classes of agents are high priority given their ease of production and availability and their toxicity at very low doses. We planned to develop an understanding of the mechanism of action of fentanyl on protein receptors, which is key to the development of cyclodextrin for sequestration of these toxic chemicals. Elucidating conditions under which zinc-based organometallic catalysts most efficiently degrade organophosphorus agents provides insight into the development of effective metallocyclodextrins for decontamination. While the cyclodextrins themselves are important for mitigating organophosphorus- and fentanyl-specific threats, the development, demonstration, and validation of an integrated design approach capable of optimizing arbitrary host–guest complexes was the central focus of our research.
Scientific Approach and Accomplishments
Synthesis of Fentanyl and Analogs
We completed the alternate and optimized syntheses of the parent opioid fentanyl and its analogs. After optimization studies were carried out for each synthetic step, the published routes exhibited high-yielding transformations leading to these powerful analgesics. The general three-step strategy produced a panel of four fentanyls in excellent yields (73–78%) along with their more commonly encountered hydrochloride and citric acid salts. The strategy offers the opportunity for the gram-scale, efficient production of these opioid alkaloids.
Kinetics and Speciation of Paraoxon Hydrolysis by Zinc(II)–Azamacrocyclic Catalysts
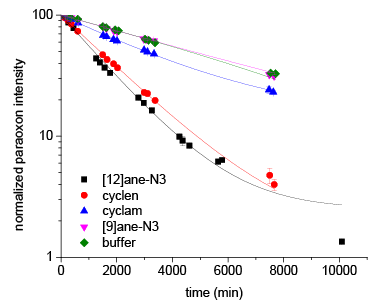
In this work, we investigated four zinc(II)-azamacrocyclic complexes for their ability to catalyze the hydrolysis of the toxic organophosphate pesticide diethyl paraoxon. Of the four complexes studied, zinc(II)-1,5,9-triaza-cyclododecane was found to be the most effective catalyst with a pseudo-first order reaction rate of k = 6.08 ± 0.23 x 10-4 min−1. Using phosphorus-31 nuclear magnetic resonance spectroscopy, we identified the two products diethyl phosphate and ethyl (4-nitrophenyl) phosphate for both catalyzed and background hydrolysis of paraoxon. Reaction rate and selectivity for forming the non-toxic diethyl phosphate correlated with the catalyst’s pKa, which is a constant indicating the strengths of acids. We found that background hydrolysis at elevated reaction temperatures (50°C) displayed no preference for diethyl paraoxon over that of ethyl (4-nitrophenyl) phosphate, despite substantial differences between the pKa values of the two leaving groups (ethoxide versus 4-nitrophenoxide anions). Kinetic rates for catalytic hydrolysis displayed an overwhelming propensity for diethyl paraoxon formation and suggest the importance of steric restrictions on transition state structure, namely a concerted arrangement of the azamacrocycle in opposition to the bulky 4-nitrophenoxy group.
Figure 1 shows the decrease in the phosphorus-31 signal intensity of paraoxon associated with the degradation of paraoxon by the four catalysts under study. Paraoxon degradation in the presence of the zinc(II)-1,5,9-triaza-cyclododecane catalyst occurred rapidly, compared to the other catalysts and the buffer. The cyclen-based complex displayed a slightly lower activity, while the cyclam-based complex displayed a small, but still enhanced activity.
Solution-State Structure and Affinities of Cyclodextrin:Fentanyl Complexes by Nuclear Magnetic Resonance Spectroscopy and Molecular Dynamics Simulation
We also investigated cyclodextrins for their ability to form inclusion complexes with the analgesic fentanyl and three similar molecules: acetylfentanyl, thiofentanyl, and acetylthiofentanyl. Stoichiometry, binding strength, and complex structure were revealed through nuclear magnetic resonance techniques and discussed in terms of molecular dynamics simulations. We found that β- cyclodextrin is generally capable of forming the strongest complexes with the fentanyl panel. We used two-dimensional nuclear magnetic resonance data and computational chemical calculations to derive solution-state structures of the complexes. Binding of the fentanyls to the cyclodextrins occurs at the amide phenyl ring, leaving the majority of the molecule solvated by water, an observation common to all four fentanyls.
Two-dimensional data using rotating frame nuclear Overhauser effect spectroscopy (ROESY) was employed to determine the proper choice of reporter protons in the fentanyl molecule. Figure 2(a) shows letter designations that have been assigned to the base fentanyl protons, while Figure 2(b) shows the ROESY spectrum for β- cyclodextrin:fentanyl–hydrogen-chloride. The presence of cross-peaks between protons on the two molecules demonstrates definitively that association occurs, as the cutoff distant for the presence of ROESY peaks is roughly 5 to 6 Å. Particularly strong correlations are observed between protons on the amide end of the fentanyl and the interior H3 and H5 protons of cyclodextrin. Specifically, all five amide phenyl protons, labelled k to m in Figure (2a), have observable correlations with H3 and H5. The propionyl methyl and methylene groups, labelled i to j in Figure 2(a), also correlate to these protons, though more weakly. These peaks indicate that the amine half of fentanyl is buried within the cyclodextrin core. The essentially nonexistent cross-peaks between H3 and H5 and any protons on the phenethyl side chain of fentanyl lead us to conclude that it dangles above the H2/H3 rim of cyclodextrin and experiences relatively unrestricted mobility.
Discovery of Cyclodextrins with Even Higher Affinity for Fentanyl
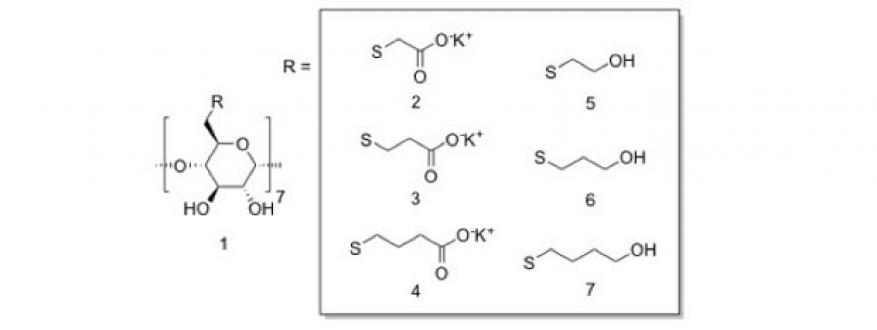
In the follow-up work to our studies above, which yielded cyclodextrins exhibiting modest equilibrium binding affinities (K of approximately 100 to 200 M−1) for fentanyl, we evaluated a library of newly synthesized cyclodextrin possessing extended thioalkylcarboxylate or thioalkylalcohol moieties. This library turned out to display remarkable affinity for fentanyl (K = 66,500 M−1), the largest value reported for such complexes to date. Nuclear magnetic resonance experiments compounded with molecular dynamics simulations suggested an unexpected binding behavior where fentanyl binds in one of two distinct energetically favorable orientations (Figure 3). Binding energies derived from computational work were found to correlate strongly with affinities derived from nuclear magnetic resonance. These correlations signified the utility of this computational work as a predictive tool for the discovery of superior cyclodextrin binders in future follow-on work of the project. The significant performance of these host molecules portends their application as medical countermeasures for opioid exposure, as biosensors, and in other forensic platforms. The synthesized cyclodextrins are shown in Figure 3.
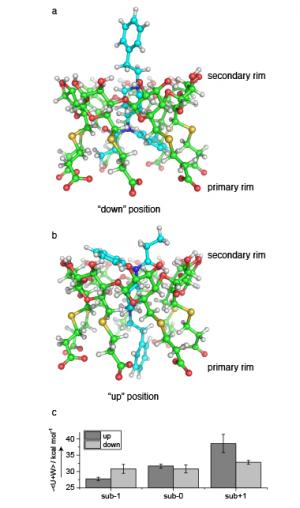
To visualize the complexes studied in the project, we conducted molecular dynamics simulations involving two distinct fentanyl orientations within the charged cyclodextrin, however, both fentanyl orientations within the cavity were shown to be favorable with relatively small differences in energies between the two possible conformations (0.8 to 5.8 kcal mol−1), as shown in Figure 4(c). In the case of suβ−1, the orientation with the amide half of fentanyl pointed “down” toward the anionic chain ends is energetically favored over fentanyl in the opposite direction, as shown in Figure 4(a). Figure 4(b) shows that for suβ+1, the reversed configuration is favored with the amide half pointed “up” toward the unmodified secondary rim of cyclodextrin. For suβ−0, the two conformations are roughly equally favorable.
Accomplishments
During the course of this project, we:
- Obtained data on the binding affinities of a number of commercially available cyclodextrins and fentanyls. The binding constants for fentanyl and other congeners were obtained for their cyclodextrin complexes.1
- Discovered an efficient route to fentanyl and analogs thereof.2
- Initially identified a panel of zinc(II)-based azamacrocyclic scaffolds capable of catalyzing the destruction of organophosphorus-based nerve agents. The catalytic nature of these scaffolds was demonstrated using paraoxon as a test organophosphorus-based agent. With the aid of one- and two-dimensional nuclear magnetic resonance spectroscopy and phosphorus-31 nuclear magnetic resonance, we were able to identify two scaffolds that catalyzed the degradation of paraoxon, effectively thus providing support to their potential use as entities capable of degrading chemical warfare agents.3
- Identified better cyclodextrin scaffolds for fentanyl and its analogs. To this end, we identified and synthesized more complex cyclodextrins that can bind the drug with an affinity that is over 100 times that of commercially available cyclodextrins. These new thiocarboxylate-based cyclodextrins possess great affinity for fentanyl. Because of their nontoxic nature, these cyclodextrins could be potentially used to capture the drug from surfaces and contaminated environmental samples, and as a medical countermeasure against a drug overdose.
- Discovered that the same panel of zinc(II)-based azamacrocyclic scaffolds used against paraoxon also work relatively well against the nerve agent O-ethyl S-[2-(diisopropylamino)ethyl] methylphosphonothioate (VX). Although they destroy the nerve agent stoichiometrically, they carry out its destruction in a region-selective manner yielding a nontoxic byproduct instead of the equally toxic EA-2192 byproduct.
The technologies developed were submitted as LLNL records of invention, and a total of three patent applications were produced from these records.4-7
Impact on Mission
Our strategy focused on the development of physical and medical countermeasures against fentanyls and organophosphorus nerve agents, which supports the Laboratory's strategic focus area in chemical and biological security for the rapid mitigation of evolving and unknown threats. This project also supported the LLNL core competency in bioscience and bioengineering by evolving an integrated computational and experimental capability for the rapid discovery of highly effective, broadly applicable scaffolds. This effort provides a foundation for future progress in environmental remediation technologies, exposure detection capabilities, and the production of advanced materials for use against known and emerging threats.
Conclusion
Our project team accomplished the goals initially set out for this project. Among those goals, we discovered and synthesized a powerful cyclodextrin core with high affinity for fentanyl that now opens new opportunities in scientific fields dealing with this drug such as medical countermeasures, decontamination protocol development, medical treatment and antidote development, and forensic science applications. The technologies we developed with this research resulted in three patent applications. We will continue to work with this newly identified scaffold and test its potential in binding other classes of drugs utilizing our established experimental and computational methods.
References
- Mayer, B. P., et al., “Solution-state structure and affinities of cyclodextrin:fentanyl complexes by nuclear magnetic resonance spectroscopy and molecular dynamics simulation.” J. Phys. Chem. B 120(9), 2423 (2016). LLNL-JRNL-680159. http://dx.doi.org/10.1021/acs.jpcb.5b12333
- Kennedy, D. J., et al., “Kinetics and speciation of paraoxon hydrolysis by zinc(II)-azamacrocyclic catalysts” Inorg. Chim. Acta 436, 123 (2015). LNL-JRNL-663756. http://dx.doi.org/10.1016/j.ica.2015.07.035
- Valdez, C. A., R. N. Leif, and B. P. Mayer, “An efficient, optimized synthesis of fentanyl and related analogs” PLoS ONE 9(9), e108250 (2014). LLNL-JRNL-656516. http://dx.doi.org/10.1371/journal.pone.0108250
- Valdez, C. A., B. P. Mayer, and E. Y. Lau, Design of homopiperazine-containing catalysts for the destruction of organophosphorus-based compounds, Lawrence Livermore National Laboratory Record of Invention IL-12892. (Patent application filed 2016).
- Valdez, C. A., B. P. Mayer, and E. Y. Lau, Click chemistry approach to metal complexes based on the bis(2-pyridylmethyl)amine scaffold: Synthesis and potential applications, Lawrence Livermore National Laboratory Record of Invention IL-1289. (Patent application filed 2016).
- Mayer, B. P., C. A. Valdez, and D. J. Kennedy, Modified cyclodextrins for the selective sequestration of fentanyls: Synthesis of “Subetadex” and related compounds and their potential applications, Lawrence Livermore National Laboratory Record of Invention IL-13067. (Undergoing review for patent filing 2016).
- Kennedy, D. J., et al., Iodide-mediated dealkylation of tributylphosphate, Lawrence Livermore National Laboratory Record of Invention IL-13074. (Patent application filed 2016).
Publications and Presentations
- Hok, S., et al., Improved and optimized syntheses of fentanyl and related analogs. (2015). LLNL-POST-675520.
- Kennedy, D. J., et al., “Kinetics and speciation of paraoxon hydrolysis by zinc(II)-azamacrocyclic catalysts” Inorg. Chim. Acta 436, 123 (2015). LNL-JRNL-663756. http://dx.doi.org/10.1016/j.ica.2015.07.035
- Kennedy, D. J., et al., Low-temperature green method for the chemical degradation of tributylphosphate. 252nd American Chemical Soc. National Mtg. and Exposition, Philadelphia, PA, Aug. 21–25, 2016. LLNL-ABS-700578.
- Mayer, B. P., C. A. Valdez, and D. J. Kennedy, Forensic analytical applications of designer cyclodextrin-modified magnetic nanoparticles for extraction of fentanyl and its analogues. 252nd American Chemical Soc. National Mtg. and Exposition, Philadelphia, PA, Aug. 21–25, 2016. LLNL-ABS-686331.
- Mayer, B. P., et al., “Solution-state structure and affinities of cyclodextrin:fentanyl complexes by nuclear magnetic resonance spectroscopy and molecular dynamics simulation.” J. Phys. Chem. B 120(9), 2423 (2016). LLNL-JRNL-680159. http://dx.doi.org/10.1021/acs.jpcb.5b12333
- Valdez, C. A., R. N. Leif, and B. P. Mayer, “An efficient, optimized synthesis of fentanyl and related analogs” PLoS ONE 9(9), e108250 (2014) . LLNL-JRNL-656516. http://dx.doi.org/10.1371/journal.pone.0108250
- Valdez, C. A., et al., Kinetic studies using 31P-NMR on the base-mediated breakdown of VX in the presence of zinc(II) complexes. 252nd American Chemical Soc. National Mtg. and Exposition, Philadelphia, PA, Aug. 21–25, 2016. LLNL-ABS-700597.


![14erd048 fig3 620.jpg Figure 2. (a) structure of the protonated fentanyl with protons labelled alphabetically and (b) proton−proton roesy spectrum for 1:2 [b-cyclodextrin]:[fentanyl hydrogen-chloride], showing only the important chemical shift regions.](https://ldrd-annual.llnl.gov/sites/ldrd_annual/files/styles/scaled_877w/public/2020-10/14erd048_fig3_620.jpg?itok=xlpvH5NN)