Nicholas Fischer (17-SI-002)
Executive Summary
We are developing a fully validated and functional first-of-its-kind model of the central nervous system that recreates the human brain's microenvironment and architecture. Our work will address fundamental questions about complex neuronal interactions as they pertain to chemical exposures and associated countermeasures, as well as disease and its mitigation.
Project Description
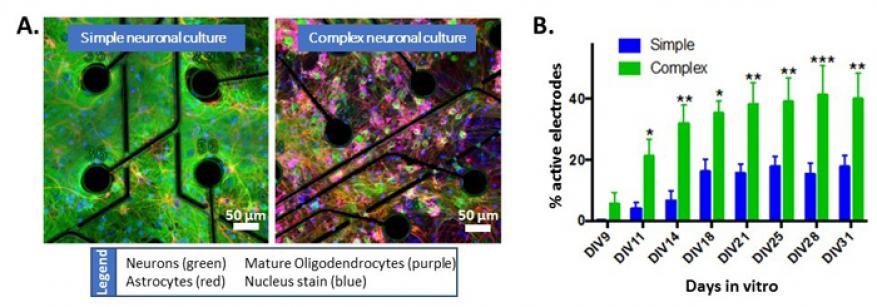
Understanding the structural complexity, exposure susceptibility, and mechanisms of disease development in the human brain are critical for creating rational interventions for neurological impairment and illness. The negative impact on the central nervous system of widespread therapeutic treatments (chemotherapeutics) and chemical toxins (pesticides and chemical warfare agents) requires an understanding of neuronal-injury mechanisms and development of modalities to mitigate adverse effects. Neurological damage and diseases cause emotional burdens and a decrease in the quality of life, and impact the U.S. economy with billions of dollars in direct healthcare costs annually. Understanding the effects of neurotoxic insult and neurodegenerative disease development requires a human brain model to analyze molecular and cellular interactions. Animal models represent the sole in vivo model for nervous system studies, but species-specific differences prevent translation to humans. Simple cell-culture systems provide basic insights into molecular mechanisms but are too far removed from the complex in vivo microenvironment. We are using the Livermore-developed in vitro chip-based human investigational platform to study neuronal function in a physiologically relevant human-based model of the central nervous system that is fully capable of interrogation (see figure). This platform incorporates four human-tissue systems: the peripheral nervous system, the central nervous system, the blood-brain barrier, and cardiac tissue. Collaborating with Stanford University, Los Alamos National Laboratory, and Livermore's Additive Manufacturing Group, we plan to increase the cellular and architectural complexity of the current platform to understand differences in human neural-network development, mechanisms of disease, and responses to chemical stimuli in simple and complex neuronal cultures.
Our goal is to develop a fully validated and functional first-of-its-kind in vitro system that recapitulates the cellular composition and spatial organization of key brain regions, thus providing unprecedented insight into the mechanistic workings of the human brain. We will assess neuronal responses to pertinent therapeutics and chemicals in healthy and diseased tissue models, using engineered human two- and three-dimensional platforms that mimic in vivo brain architecture and complexity. This research will address fundamental scientific questions about the function and network interaction of complex neural tissue. Our project deliverable will be a system that integrates the Laboratory's blood-brain barrier model with the central nervous system device, providing in-depth analysis of multiple-neuron interactions as they pertain to chemical exposures (and countermeasures), disease, and changes in their microenvironment.
Mission Relevance
This project supports the DOE's objective of delivering scientific discoveries and tools that transform our understanding of nature. It also enhances the Laboratory's bioscience and bioengineering core competencies by enabling research into basic cellular mechanisms and pathways, and supports the chemical and biological security research-and-development challenge by enabling examination of toxic effects of a terrorist attack employing chemical or biological agents.
FY17 Accomplishments and Results
In FY17 we (1) increased the complexity of rodent two-dimensional neuronal systems; (2) began collecting data to enable a complete validation dataset of in vivo comparisons by establishing baseline neuronal activity and viability; (3) validated the blood-brain barrier model, which is being optimized for flow experiments to enable the integration of the barrier with complex neuronal cultures; (4) streamlined data analysis for rapidly analyzing large datasets; and (5) initiated experiments to compare activity and transcription profiles of different human neuronal-cell populations.
Publications and Presentations
Lam, D., et al. "Development and Characterization of Primary Neuronal-Glial Networks." Asilomar Bioelectronics Symposium, Pacific Grove, CA, USA. September 17-20, 2017. LLNL-POST-738164.
Moya, M., et al. "Three-Dimensional Dynamic Blood-Brain Barrier Model." Tissue Engineering & Regenerative Medicine: Personalized and Precise Science, Engineering, and Translation, San Diego CA, December 11-14, 2016. LLNL-PRES-713825.