Patrick G. Campbell (16-LW-013)
Abstract
Carbon capture and sequestration (CCS) technology is an approach to limit the rise of carbon dioxide (CO2) in the atmosphere, but the carbon-capture step is economically unfeasible using current CO2-separation technology. We investigated a novel dual-phase CO2-separation membrane based on molten hydroxide supported by a porous ceramic material. The membrane operates at flue-gas temperatures (300-650°C) and can dramatically reduce the energy and infrastructure costs of CO2 separation from fossil-fuel power plant flue gas. To produce this membrane, we developed a robust porous ceramic material with tunable properties (density, pore morphology, etc.) that can be cast into molds, extruded, or utilized in additive manufacturing (AM) processes (such as 3D printing). We demonstrated reversible CO2 absorption in molten-hydroxide mixtures in the presence of water vapor. We measured membrane performance under realistic operating conditions and observed CO2 permeability that was 10 times greater than that of the best values for dual-phase membranes based on molten carbonate liquid phase.
Background and Research Objectives
Greenhouse gases such as carbon dioxide (CO2) and methane are released into the atmosphere through a variety of natural causes and human activities. Since the dawn of the industrial revolution 250 years ago, CO2 released from the burning of fossil fuels for energy has increased the concentration of CO2 in the atmosphere by nearly 50 percent. The current atmospheric CO2 concentration (407 ppm) is higher than at any time in the last 20 million years (Houghton et al. 2001). The increase in atmospheric CO2 has caused the average global temperature to rise by approximately 1 °C since record keeping began in 1880. If CO2 levels continue to rise at the current rate, the Earth’s average temperature will increase by another 3.5 °C by the end of the 21st century (Pachauri and Reisinger 2007). Along with the impact of changing weather patterns and rising sea levels, other negative effects of increased CO2 in the atmosphere include ocean acidification, which can harm marine ecosystems, and melting permafrost, which could release large amounts of additional carbon into the atmosphere and further accelerate global warming.
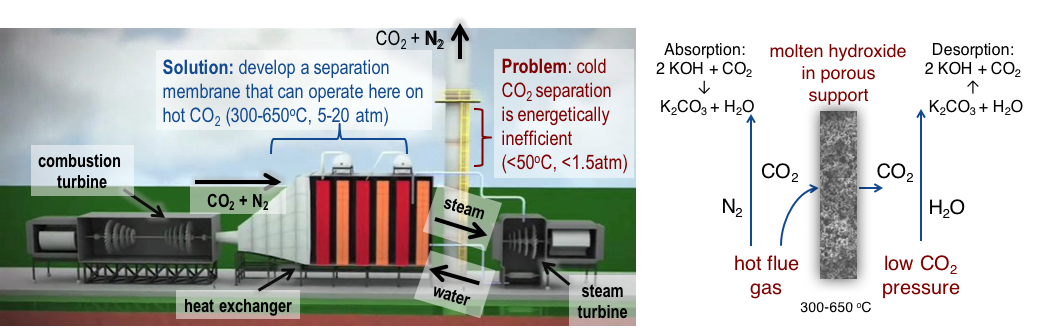
One approach to limit the rise of atmospheric CO2 is to capture it at the source of emission and sequester it underground or convert it to mineral form. This is known as carbon capture and sequestration (CCS). This approach works best for large, fixed sources of CO2 emissions, such as power plants and industrial facilities. Currently, emissions from large point sources constitute more than half of the total US carbon emissions. Fossil-fuel power plants are the single largest source of anthropogenic carbon emissions world-wide. Typical fossil-fuel power plants produce CO2 as a relatively minor constituent in a mixture of exhaust gasses consisting mainly of N2 and H2O. For efficient storage, the CO2 must be separated from this mixture at the carbon-capture (CC) step, which is challenging due to the dilute concentration of CO2 and its low reactivity. Once separated, the CO2 is piped to an appropriate site for the sequestration step, which presents its own set of challenges, such as finding suitable geology for underground storage, supplying sufficient pressure for deep well injection, and long-term leak monitoring. This project was focused on the CC step.
The state-of-the-art technology for CC is amine-gas treatment. In this process, flue gas that has cooled to nearly ambient temperature (<50 °C) is bubbled through a solution containing basic amines (typically monoethanolamine, MEA), which react with the acidic CO2, forming an acid-base adduct. When the solution becomes saturated with CO2, it is pumped to a different reactor where it is heated above 100 °C to release the CO2 for sequestration and regenerate the sorbent. Carbon capture using MEA has a number of drawbacks including low CO2 loading capacity (the weight of CO2 absorbed per kilogram of absorbent), high equipment-corrosion rates, and sorbent degradation by SO2, NO2, HCl, HF, and O2 in flue gas. However, the single largest issue with amine-gas treatment is the extremely high energy required for the regeneration step, which can consume between 25 and 40 percent of the total energy output of the power plant (Yeh et al. 2004). Moreover, the additional infrastructure required by an amine-gas treatment system can double the operating cost of a power plant.
Membrane-gas separation is an appealing alternative to amine-gas treatment. Membrane separation requires much less energy than sorbent-based separation because the separation process is spontaneously driven by the difference in CO2 partial pressure on either side of the membrane and there is no regeneration step. Recently there has been a great deal of effort to develop highly selective polymer membranes for CO2 separation (Shekhawat et al 2003; Kim and Lee 2013). However, polymer membranes operate at low temperatures (typically less than 100 °C), which lowers the rate of CO2 diffusion across the membrane. To overcome the slow diffusion rate, the membranes must be very thin, which impacts mechanical properties and increases cost. Additionally, since polymer membranes separate flue-gas constituents based on the difference in their diffusion rates, they are not perfectly selective for CO2.
In contrast to polymer membranes, dual-phase membranes employ a liquid phase supported by a porous solid phase. The liquid is held in the membrane's pores by capillary force. Dual-phase membranes in which the liquid phase is a mixture of molten carbonate salts have been investigated for CO2 separation (Wade et al. 2011; Lu and Lin 2013; Rui et al. 2012; Xing et al. 2015). In order to achieve acceptable CO2 flux, these membranes must operate at temperatures above 650°C, which is higher than the flue-gas temperature in the combustion turbine or boiler. The high temperature requirement is due to the membrane chemistry, which enables perfect selectivity for CO2 (transported as carbonate, CO3, during the molten phase), but is rate-limited by oxide (O2-) transport in the opposite direction during the solid phase. Solid-phase O2- transport is very slow at temperatures below 650°C.
To lower the operating temperature for dual-phase membranes while improving overall CO2 flux, the oxide ion (O2-), which is required to react with CO2 to form carbonate (the species that is transported across the membrane), should also be transported during the liquid phase. Replacing the molten-carbonate phase with molten hydroxide would achieve this goal, but the reaction of CO2 with hydroxide has traditionally been viewed as irreversible. Recently, however, several research groups working in the area of molten-carbonate fuel cells have independently reported that CO2 absorption by molten hydroxides is reversible above 250 °C in the presence of sufficient water vapor (steam) (Zecevic et al. 2005; Nunoura et al. 2007; Guo et al. 2013). This discovery could enable a dual-phase membrane based on molten hydroxides (Figure 1) to operate in the flue-gas temperature range of 300-650°C, using steam to release CO2 from the membrane. The physical configuration and the chemical process of this membrane are illustrated in Figure 1.
The objective of this project was to develop a dual-phase CO2 separation membrane using a molten-hydroxide liquid phase supported in a porous material. To meet this objective, we developed a porous support material, demonstrated reversible CO2 absorption by hydroxide melts under varied temperature and steam pressure regimes, and tested dual-phase membranes under realistic operating conditions. We successfully produced membranes with very high CO2 selectivity and recorded permeability values an order of magnitude greater than the best values documented in the literature. The novel porous ceramic support materials developed as part of this project are mechanically robust and compatible with the system's high operating temperatures and corrosive molten phase. The process for producing these materials can be adapted to utilize additive manufacturing (AM) techniques such as direct-ink writing (DIW) and projection micro-stereo lithography (PµSL), which can provide more control over membrane structure and pore morphology, and enable better CO2 separation performance. The need for heat and steam together led to the development of a humidified tube furnace at Lawrence Livermore National Laboratory, which is a new capability that has already been utilized by other Livermore projects.
Scientific Approach and Accomplishments
Porous Solid-Support Material
The porous solid-support material for the membranes must be stable in the presence of highly caustic molten-hydroxide mixtures at temperatures above 650°C and have interconnected pores with the correct morphology. We evaluated a number of commercially available porous ceramic and metal materials, including alumina, which dissolved in the molten hydroxide; stainless steel, which showed signs of corrosion; and silicon carbide, which oxidized and leaked. Other materials that are compatible with the membrane operating conditions, including CeO2 and yttria-stabilized zirconia (YSZ), were not commercially available in the right form factor with the correct pore geometry. Since the material used for the solid support was crucial to the success of our project, we produced our own ceramic support material with the appropriate properties.
The method we developed enables remarkable control over material properties (such as density, pore size, and pore volume) by making simple adjustments to the reaction conditions. It enables porous ceramic parts to be rapidly produced in large quantities and in arbitrary shapes and sizes by casting into molds or extruding cylinders and tubes. It is also compatible with AM techniques such as PµSL and DIW, which can enable rapid prototyping of optimized membrane designs as well as provide a new AM “feed stock” to expand the applicability of AM into other fields of research.
Reversible CO2 Absorption in Hydroxide Melts
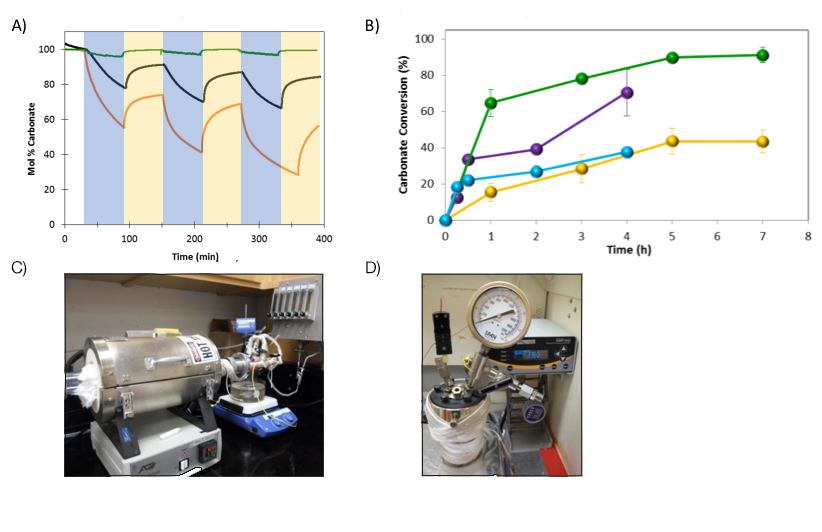
To prove the feasibility of CO2 separation by dual-phase membranes based on molten-hydroxide chemistry, we needed to evaluate the reversibility of CO2 absorption by molten hydroxides under various temperature and humidity conditions. We were able to demonstrate reversibility using three different methods: (1) thermogravimetric analysis (TGA), (2) saturated steam in a tube furnace, and (3) high-pressure steam in a pressure reactor. We used a mixture of Li/Na/K hydroxide to achieve the lowest melting point and highest dehydration temperature. For the tube furnace and pressure-reactor experiments, the hydroxide mixture was loaded into porous YSZ support materials, which were then crushed and treated with N2/CO2 to convert the hydroxides into carbonates. Titration was used to determine the extent of conversion before and after humidity experiments.
To improve conversion at lower temperatures using increased water-vapor concentration, we developed a humidified tube furnace (Figure 2C). By passing pre-heated N2 sweep gas though a flask of boiling water, we were able to introduce saturated steam into the tube furnace. To vary the steam pressures, we used a pressure reactor filled with a calibrated amount of liquid water to pressurize the steam once the vessel was at temperature (Figure 2D). Experimental results are presented in Figure 2D. Using steam in the tube furnace at the right pressure, temperature, and time enabled nearly complete conversion of carbonate to CO2, while at other temperatures, the conversion was limited to approximately 40 percent. By increasing the steam pressure, more than 70 percent of the carbonate could be converted to CO2 in a shorter time and lower temperature. These results highlight the crucial role steam concentration plays in the reversibility of CO2 absorption by molten hydroxides.
Membrane Evaluation under Realistic Operating Conditions
With the membrane-support material and molten phase in place, the last step to achieve the project objectives was to evaluate membrane performance under realistic operating conditions. To perform this work, we collaborated with Sangil Kim at the University of Illinois, Chicago. His research group has the expertise and equipment for high-temperature membrane characterization. We utilized their custom-designed high-temperature permeation cell that can be placed into a furnace while different mixtures of feed and sweep gasses are introduced. The sweep gas is humidified by passing pre-heated helium through a beaker of boiling water. After passing by the membrane, the sweep gas enters a gas chromatograph to determine the concentration of CO2.
Membranes were tested for leaks before single (100 percent CO2) and binary mixed gas (50:50 CO2/N2)-permeation experiments were conducted. Comparing our membrane performance to the best dual-phase membranes documented in the literature (which all utilize molten-carbonate liquid phases), the molten-hydroxide dual-phase membranes have CO2 permeability that is an order of magnitude higher than that of the best comparable membrane (Lu and Lin 2013; Wade et al. 2011; Rui et al. 2012); and Xing et al. 2015).
Impact on Mission
The findings of our project's research support the mission and goals of the Lawrence Livermore National Laboratory's Global Security E-Program to develop carbon-mitigation technologies. Our work also supports the development of efficient and cost-effective CCS technologies, a key goal of the DOE's Carbon Storage Program. Additionally, the newly developed capability to additively manufacture porous ceramics will help maintain the Laboratory at the forefront of AM technology. The ability to engineer porous ceramic materials with precise control of product characteristics will facilitate new developments in diverse applications in filtration, catalysis, and structural materials. Other capabilities developed for this project, such as humidified and pressurized furnaces and reactors, analytical methods, and membrane-separation expertise, will continue to be of value to various Laboratory program areas.
Conclusion
We developed a dual-phase CO2 separation membrane based on a molten hydroxide supported in a porous ceramic material, which can operate at flue-gas temperatures. Our membrane demonstrated CO2 permeability 10 times greater than the best values reported in the literature. The high-permeability and high-temperature operation makes this technology significantly more energy efficient than the current state-of-the-art CO2-separation technology, amine-gas treatment. Our membrane technology could make CCS more economically feasible and ultimately lead to reduced CO2 emissions into the atmosphere.
References
Campbell, P.G., et al. 2016. Molten Hydroxide Membrane for Separation of Acid Gases from Emissions. US Patent Application No. 15/159,681; filed 19 May 2016.
Guo, L., et al. 2013. “Development of a Low Temperature, Molten Hydroxide Direct Carbon Fuel Cell.” Energy & Fuels 27 (3): 1712–19. doi:10.1021/ef302100h.
Houghton, J.T., et al, eds. 2001. Climate Change 2001: The Scientific Basis. Cambridge University Press, Cambridge, UK doi: 10.1002/joc.763.
Kim, S., and Y.M. Lee. 2013. “High Performance Polymer Membranes for CO.” Current Opinion in Chemical Engineering 2 (2). Elsevier Ltd: 238–244. doi: 10.1016/j.coche.2013.03.006.
Lu, B., and Y.S. Lin. 2013. “Synthesis and Characterization of Carbonate-Ceramic Dual-Phase Membranes for Carbon Dioxide Separation.” Journal of Membrane Science 444 (C). Elsevier: 402–11. doi: 10.1016/j.memsci.2013.05.046.
Nunoura, T., et al. 2007. “Performance of a First-Generation, Aqueous-Alkaline Biocarbon Fuel Cell.” Industrial & Engineering Chemistry Research 46 (3): 734–744. doi: 10.1021/ie061202s.
Pachauri, R.K., and A. Reisinger, eds. 2008. Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva, Switzerland: IPCC.
Rui, Z., et al. 2012. “Ionic Conducting Ceramic and Carbonate Dual Phase Membranes for Carbon Dioxide Separation.” Journal of Membrane Science 417–418 (174–182). doi: 10.1016/j.memsci.2012.06.030.
Shekhawat, D., et al. 2003. A Review of Carbon Dioxide Selective Membranes: A Topical Report, DOE/NETL-2003/1200. doi: 10.2172/819990.
Wade, J.L., et al. 2011. “Composite Electrolyte Membranes forr High Temperature CO2 Separation.” Journal of Membrane Science 369 (1-2): 20–29. doi: 10.1016/j.memsci.2010.10.053.
Xing, W., et al. 2015. “Steam-Promoted CO2 Flux in Dual-Phase CO2 Separation Membranes.” Journal of Membrane Science 482: 115–119. doi: 10.1016/j.memsci.2015.02.029.
Yeh, J.T., et al. 2004. “Absorption and Regeneration Studies for CO2 Capture by Aqueous Ammonia.” Third Annual Conference on Carbon Capture & Sequestration, Alexandria, VA, May 3–6, 2004, presentation.
Zecevic, S., et al. 2005. “Direct Electrochemical Power Generation From Carbon in Fuel Cells with Molten Hydroxide Electrolyte.” Chemical Engineering Communications 192 (10-12): 1655–1670. doi: 10.1080/009864490896241.
Zheng, X., et al. 2014. “Ultralight, Ultrastiff Mechanical Metamaterials.” Science 344 (6190): 1373–1377. doi: 10.1126/science.1252291.
Publications and Presentations
Campbell, P.G., et al. 2016. “Porous Solid Supports for Dual-Phase Liquid/Solid Membranes." North American Membrane Society Meeting, Bellevue, WA, 21–25 May, invited presentation.
Ceron, M.R., et al. 2017a. “Dual-Phase Membranes for Flue Temperature CO2 Separation” Invited Talk- Materials for Energy and Emerging Technologies (MEET)-UTEP, 17-18 May-2017. LLNL-PRES-731030.
——— 2017b. “Dual-Phase Membranes for Flue Temperature CO2 Separation” Poster- Institutional Postdoc Symposium- LLNL, 14 Jun-2017. LLNL-POST-732497.
——— 2017c. “Dual-Phase Membranes for Flue Temperature CO2 Separation” Poster- International Congress on Membranes and Membrane Processes, San Francisco, CA, 29 Jun 4 Aug 2017. LLNL-POST-732497.
——— 2017d. “Dual-Phase Membranes for Flue Temperature CO2 Separation” Presentation- Research Slam- LLNL, 10 Aug-2017 LLNL-PRES-736515.