Swanee Shin (15-ERD-063)
Abstract
We performed a series of experiments to study the characteristics of an effective template for solid hydrogen nucleation. Zinc stood out among several materials with comparable size and shape. Nucleation occurred on top of sharp features—such as grain boundaries and cracks—but our attempts to fabricate or replicate those structures proved unsuccessful. The variations in the supercooling values measured for comparable samples revealed that templated nucleation of solid hydrogen is highly sensitive to experimental conditions.
Background and Research Objectives
The energy source for Inertial Confinement Fusion (ICF) is a nuclear fusion reaction of a mixture of hydrogen isotopes (deuterium–tritium), solid layers of which are used to coat the inside wall of a thin-walled spherical ablator shell at the National Ignition Facility (NIF). However, routinely making uniform, high-quality, solid hydrogen layers inside the ablator shell remains a challenge. Multiple solidification attempts over several days are typically needed to form an ignition-quality, single crystalline layer acceptable for ICF experiments. One of the most critical steps in making a high-quality layer was to form a solid seed (or nuclei) that further grew into a single crystal, which was often very difficult to control and characterize.
Previous research suggested that one way to improve the speed and reliability of the procedure was to place a seed producer in the ablator shell for deterministic hydrogen nucleation by capitalizing on the heterogeneous nucleation process of hydrogen in contact with the seed producing material. This so-called “templating” effect of various materials has been explored (Shin et al. 2016), and zinc was identified as one of the most promising materials to offer multiple processing and characterization options (Bernat et al. 2016). However, supercooling—the metric that measures the effectiveness as a template—exhibited large variations depending on the initial sample size, formula, and follow-up processing.
The objectives of this project were to address key questions that arise from the foundational work. We sought to
- better identify and characterize the morphological features that promote hydrogen nucleation,
- fabricate or replicate such features on zinc or on other materials,
- discover the reliability of the zinc size effect, and
- investigate cell vacuum condition effects on the results.
Scientific Approach and Accomplishments
The effectiveness of a template was quantified using the degree of supercooling below the triple point (hereafter denoted as ΔT) required for the liquid hydrogen contacting the template to solidify. A small ΔT is favorable because a good template needs to promote hydrogen solidification. We measured the ΔT below the triple point of hydrogen—either hydrogen or deuterium—required for liquid hydrogen in contact with a sample to solidify. The stage temperature was ramped down until the liquid layer solidified, and ramped up until the solid layers melted back at the triple point. Details of the experimental methods, as well as cell and cryostat designs were reported in a journal article (Shin et al. 2016).
We performed all zinc foil experiments with 50-μm thick, commercially available rolled foils. Since ΔT proved to be very insensitive to the surface chemistry, we performed no specific cleaning or treatment of the samples before or in-between experiments (Bernat et al. 2016); however, we did handle foils with a vacuum needle when moving, loading, and unloading them to minimize morphological changes. To actively make preferred nucleation sites, we created sharp features on zinc foil surfaces using an MTS XP-navbar-brand nanoindenter with a Berkovich tip (half-angle theta equaled 72.1°) and a custom-made, three-sided tip (half-angle theta equaled 20°). Both tips were used to make indents while the trench pattern was exclusively made with the three-sided tip.
Materials survey
We reported a ΔT of various template materials (Shin et al. 2016). Most of the metals showed high ΔT—greater than 100 mK—but the sample forms varied from shots to cut wafers, aerogels, and deposited films. Since zinc exhibited a wide range of ΔT depending on the size and form, we compared the ΔT of several materials that were comparable in size and form. Among various metals that had a similar size (3–5 μm) and shape (shot, pellet) including gold, zirconium, tin, silver, lead, cobalt, titanium, and aluminum, it was still zinc that stood out as the lowest ΔT of less than 50 mK. Specifically, a zinc shot and 50-μm thick foil consistently show very low ΔT of less than 20 mK. In addition, researchers observed that the low ΔT of a zinc shot and grains were preserved regardless of the surface processing that followed—such as with acid etching and atomic layer deposition (ALD) of aluminum dioxide layer (Bernat et al. 2016). We measured the ΔT of zinc and gold foils—both 3×3 mm in size and 50 μm in thickness—after an aluminum dioxide ALD, which was approximately 30 nm thick. The ΔT did not change after the ALD, which produced 15 mK for zinc and 200 mK for gold. X-ray photoelectron spectroscopy measurement results confirmed that the surfaces were completely covered with aluminum, oxygen, and carbon after ALD, and neither zinc nor gold signal was detected from the coated zinc and gold foils, respectively. These developments strongly suggested that morphological features on the zinc samples were responsible for favorable solid hydrogen nucleation.
Zinc: morphology
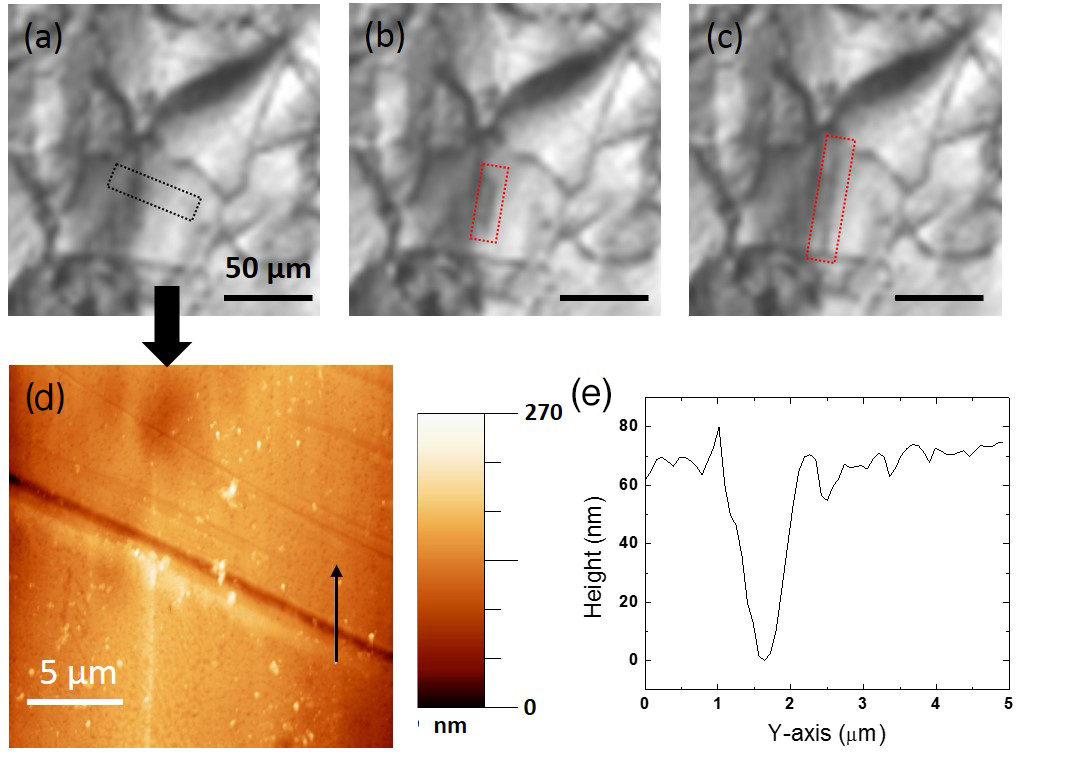
To better identify features that promote solid hydrogen nucleation, we performed solidification experiments with a polished zinc foil. During these experiments, we observed hydrogen freezing events in situ with an optical microscope. The foil was approximately 3×3 mm in size and 50 μm in thickness, and the ΔT was measured between 10–15 mK. We noted that the polishing process did not change the ΔT of the zinc foil. Since the ΔT was low, the nucleation rate was also low; therefore, only 1–2 nucleations occurred on the entire surface before the propagating growth fronts covered the whole surface. We located such nucleation sites and characterized them in situ with an optical microscope, and ex situ with an atomic force microscope. We saw solid hydrogen start to form on top of a grain boundary (not shown), and a sharp scratch that accidentally formed during the polishing process (Figure 1). It first formed a thin, solid ribbon very rapidly, and then expanded more slowly towards both sides of the ribbon in normal directions. It was an asymmetric growth pattern typically observed for solid hydrogen growth (Chernov et al. 2009). Similar nucleation and growth behaviors were observed for several different grain boundaries and cracks or scratches on multiple samples. Thus, it appeared that sharp, crack-like features acted as favorable nucleation sites. It is still not clear however which characteristics of such sites stand out among other abundant similarly-shaped sites around them, but the fact that sharp cracks promote nucleation agreed with previous nucleation studies (Sholl et al. 1970, Campbell et al. 2013). Our attempts to fabricate and replicate favorable nucleation sites are discussed below in the "Fabricate and replicate surface features" section.
Fabricate and replicate surface features
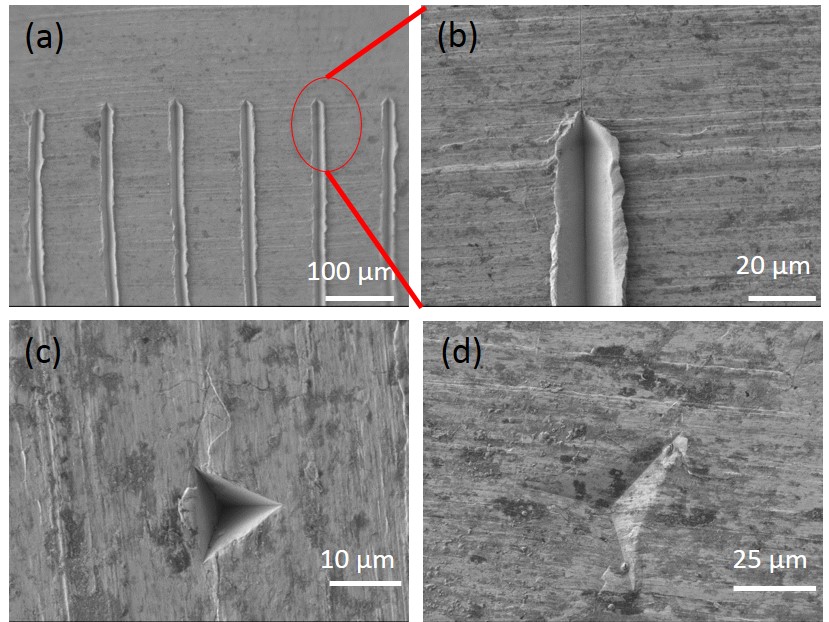
We fabricated sharp features with known geometries on a zinc foil to test the active control of preferred nucleation sites. Figure 2 shows scanning electron microscope images of the features we made on a zinc foil using nanoindentation as described earlier. The trenches in Figure 2.a and indents in Figure 2.c–d did not act as nucleation sites. Nucleation of solid hydrogen occurred away from these features on non-distinctive sites, and the ΔT was the same as non-patterned zinc foils at approximately 15 mK.
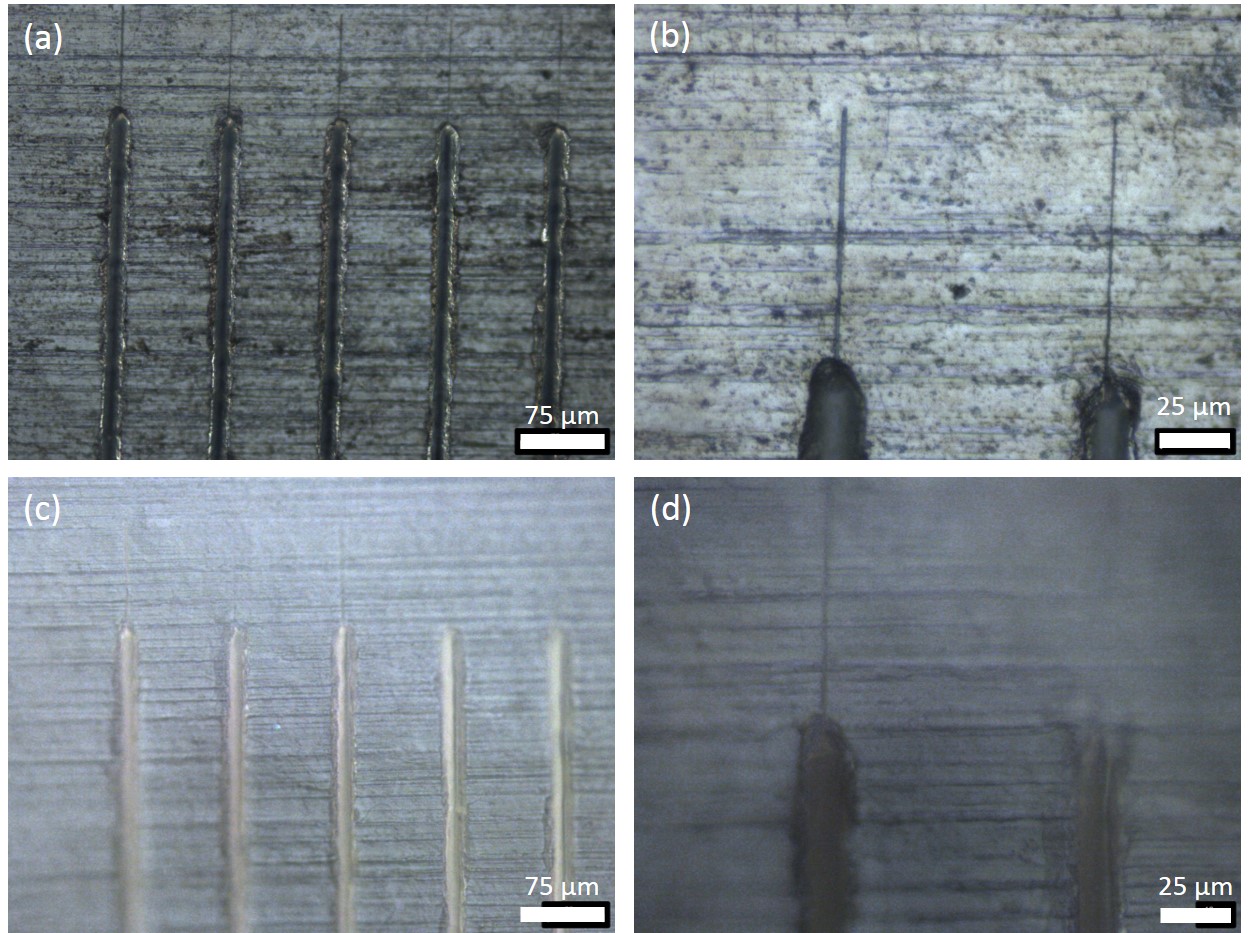
To replicate all morphological features on a zinc foil—including the ones that are responsible for low ΔT (approximately 15 mK), we made a replica of the zinc foil with trenches (Figure 2.a) using polydimethylsiloxane (PDMS, Sylgard 184 Dow Corning) to make a mold and polyurethane (PU, Norland Optical Adhesive 73) for the actual zinc replica. PDMS was mixed in a 10 (base) : 1 (curing agent) ratio, and cured at room temperature for three days. The PU was photopolymerized with UV lamp (Hg-arc lamp, 100 W, 10 cm sample to light distance) for 40 minutes. The trenches served as a “landmark” to find the same location on the zinc foil and PU replica, and to assess the effectiveness of the replication process.
Figure 3 shows optical microscope images of the zinc foil and PU replica. We noticed that not only were the major trenches of approximately 20-μm widths replicated, but other features that were approximately 1 μm in size—such as thin drag lines at the end of the trenches and horizontal lines from the foil manufacturing process—had also been well transferred to the PU sample. As reported above, we did not observe solid hydrogen nucleation from these recognizable features, but did learn to expect the majority of morphological features on the zinc foil—down to approximately 1 μm—to be transferred to the PU replica. However, the measured ΔT of that PU replica template was approximately 250 mK, which was not different from a PU control sample without replicated features. Therefore, if the feature size alone was responsible for the low ΔT observed, then the features were expected to be less than approximately 1 μm in size, such as the one in Figure 1. The critical diameter of a solid hydrogen nucleus with a ΔT of 15 mK is about 50 nm according to classical nucleation theory, and it is possible that the small, responsible features of this scale have not been properly replicated (Bernat et al. 2016).
Zinc: size effect
The sample size dependence on ΔT has an important implication for ICF target applications since most of the target components relevant to hydrogen layers are sub-millimeter (down to a few microns) in size, and do not allow large space to fit into a template. Since the increase of ΔT for smaller zinc samples (less than approximately 1 mm) was observed across non-controlled, various forms of zinc (shots, powders, films), we revisited the size effect with a systematic study (Bernat et al. 2016).
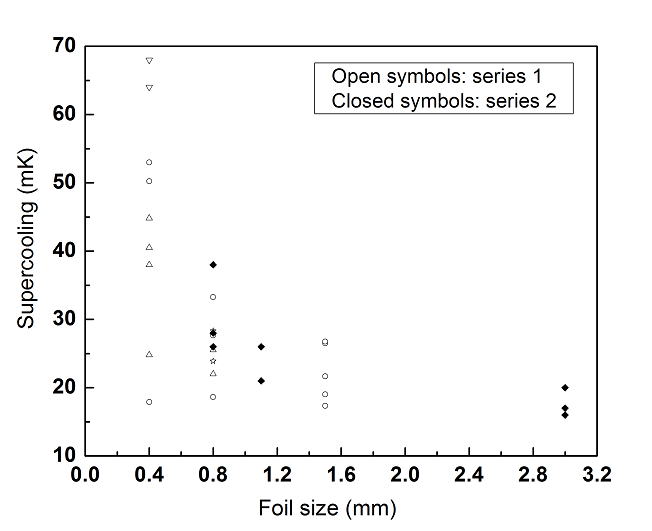
To carry out this study, we started with a zinc foil that was approximately 3×3 mm in size, and 50 μm in thickness that showed a ΔT of between 10–15 mK. The foil was subsequently cut into half, and the ΔT of each square foil of approximately 3, 1.5, 0.8, and 0.4 mm in size was measured. Several different samples with the same size were tested individually, and the same sample was also tested multiple times over independent attempts to obtain more reliable statistical data. (See Series 1 in Figure 4.) The results are plotted below in Figure 4. The average ΔT increased as the sample size decreased, which was consistent with previous observations (Bernat et al. 2016). However, there were large variations among samples with the same size and even for the same sample across different attempts. The variations were random without any systematic correlations.
To further test the size effect, another series of ΔT experiments was performed with cut zinc foils by placing different amounts of foil on an experimental cell, all at the same time . The zinc foil—initially 3 mm in size—was first cut into four pieces of 1.5×1.5 mm, then into sixteen 0.8×0.8 mm samples. (See Series 2 in Figure 4.) The results were plotted together with Series 1 in Figure 4 and showed three results. First, the ΔT had a strong dependence on the total amount of zinc, rather than the size of the individual piece. Second, the ΔT of a specific zinc piece changed depending on the total amount of zinc in the cell. Third, there were many similar competing nucleation sites on each piece of zinc foil, and preferential nucleation at a specific site was not easily reproduced.
The discovery that the ΔT of a specific zinc piece changed depending on the total amount of zinc in the cell was surprising and difficult to explain. It suggests that other factors, such as variation in cell vacuum conditions, modification of samples during handling may have had a strong influence on the results. To counter this, we made an attempt to better control the experimental conditions by first addressing the possible residual surface contamination.
Impact on Mission
This project directly supported Livermore's mission in nuclear weapons stockpile stewardship and enhanced two of the Laboratory's core competencies (high-energy-density science and advanced materials and manufacturing) by aiming to gain scientific understanding of the solid hydrogen nucleation process to improve ICF fuel layering protocol.
Conclusion
Zinc, with a low ΔT of less than 50 mK, stands out as a template for hydrogen solidification among several other materials. Experimental results suggested that fine features, such as grain boundary and cracks, promoted solid hydrogen nucleation, but we were not able to fabricate or replicate these features. A possible explanation was that zinc has sub-micron features that promote the nucleation of hydrogen, and those features were subject to passivation due to water vapor or other residual gases. The variations of ΔT among samples with the same size—and for the same sample across different attempts—revealed that templated seeding of solid hydrogen is a very delicate process that requires a better controlled environment to be useful as a seed material for ICF fuel layers.
References
Bernat, T., et al. 2016. “Zn-nucleated D2 and H2 Crystal Formation from Their Liquids.” Fusion Science and Technology 70 (2): 196-205. LLNL-JRNL-737464. doi: 10.13182/FST15-223.
Campbell, J. M., et al. 2013. “Characterization of Preferred Crystal Nucleation Sites on Mica Surfaces” Crystal Growth and Design 13 (5): 1915-1925. doi: 10.1021/cg301715n.
Chernov, A. A., et al. 2009. “Single Crystal Growth and Formation of Defects in Deuterium–Tritium Layers for Inertial Confinement Fusion” Applied Physics Letters 94 (6): 064105. doi: 10.1063/1.3080655.
Shin, S. J., et al. 2016. “Supercooling Study of Hydrogen on Template Materials to Deterministically Seed Ignition Quality Solid Fuel Layers.” Fusion Science and Technology 70 (2): 184-190. doi: 10.13182/FST15-212. LLNL-JRNL-705308.
Sholl, C. A. and Fletcher, N. H. 1970. “Decoration Criteria for Surface Steps” Acta Metallurgica 18 (10): 1083-1086. doi: 10.1016/0001-6160(70)90006-4.
Publications and Presentations
Bernat, T., et al. 2016. “Zn-nucleated D2 and H2 Crystal Formation from Their Liquids.” Fusion Science and Technology 70 (2): 196-205. LLNL-JRNL-737464. doi: 10.13182/FST15-223.
Shin, S. J., et al. 2016. “Supercooling Study of Hydrogen on Template Materials to Deterministically Seed Ignition Quality Solid Fuel Layers.” Fusion Science and Technology 70 (2): 184-190. LLNL-JRNL-705308. doi: 10.13182/FST15-212.
——— 2017a. “Hydrogen Solidification on Template Materials for Nuclear Fusion Applications.” 2017 Materials Research Society Spring Meeting and Exhibit, Phoenix, AZ, 17-21 April 2017. LLNL-POST-728699.
——— 2017b. “Materials and Morphology Study for Templated Hydrogen Solidification.” Fusion Science and Technology. Nov. 29, 2017. LLNL-JRNL-736555. doi: 10.1080/15361055.2017.1387015.