Thomas Han (13-ERD-022)
Abstract
Nanometer-scale materials can have a significant impact in advancing technology because of their unique properties. However, a key challenge that remains in the application of nanomaterials has been developing high-quality, reproducible nanomaterials in large quantities. Retaining the quality of nanomaterials while scaling up synthesis is not a trivial process, requiring many iterations and optimizations to achieve the desired results. A continuous flow-synthesis platform can significantly shorten the optimization step by quickly screening the reaction parameters to obtain the desired results. In this project, we developed a continuous synthesis platform for synthesizing nanomaterials, which we attempted to assemble in three dimensions. The dynamic controls provided by the continuous flow-synthesis system, which enable quick “on-the-fly” changes in chemicals and their concentrations as well as temperature and mixing rates, allow screening of large synthesis phases in a given system in a relatively short timescale. We chose to synthesize copper(I)-oxide crystals in different morphologies and sizes, changes that can be profoundly influenced by synthesis parameters. We have demonstrated that copper(I)-oxide nanomaterials can develop different crystal facets, morphologies, and size variations with minor synthesis parameter changes. These material attributes can impact their catalytic activities and can also be used to selectively "design" their surfaces to allow the oriented assembly of nanomaterials.
Background and Research Objectives
One of the grand challenges of materials science is to create designer materials with pre-programmed building blocks that can assemble predictively or as directed into structures with unique functions and properties.1 These designer materials could also have the ability to self-regulate or adapt their functions and properties, including electrical conductivity or elasticity or their magnetic, optical, and mechanical properties on demand. Such capabilities have the potential to lead to significant changes in the world of technology. The creation of such dynamic materials requires the understanding and the ability to control the interfacial and free-energy landscapes of the building blocks to direct their assembly from atomic to macroscopic length scales. An example of ultimate control of directed assembly is seen in organic chemistry. Both simple and complex organic molecules can be created with just four atoms: carbon, hydrogen, nitrogen, and oxygen. An enormous number of organic molecules with remarkable properties and functions can be synthesized with these few types of atoms, because of the level of control one has during their assembly. Similar approaches can be invoked at longer length scales in materials science. Nanoparticles with diverse chemical compositions, shapes, sizes, and structures can be used as “artificial atoms” to construct nano-meso-macroscopic “molecules” of nanoparticles with increasing complexity and function. If multiple nanoparticles with novel properties can be assembled systematically in any structure or order, new materials can be predictively created, and novel unforeseen properties that can be controlled and manipulated will emerge, providing a paradigm shift in materials science.
Examples of ordered nanoparticle assemblies known as nanocrystal superlattices (NSLs), have been reported.2 Their existence demonstrates the proof of concept for building macroscopic structures based on nanoparticles. However, the length scales of NSLs are relatively short (often less than a micron in two dimensions only). One of the most difficult hurdles to overcome the current limitations is the controlled assembly of nanoparticles. Currently, NSLs are formed by an empirical self-assembly process during the evaporation of solvents. This approach only provides a limited level of control. A higher level of control will be afforded by the in-depth understanding of the particle-to-particle interactions to direct their assembly to higher ordered structures in longer length scales. One way to achieve this goal is to synthesize nanomaterials with unique crystalline facets that can be selectively designed to control their assembly. We synthesized nanomaterials with different facets to control their surface chemistry for assembly with the ultimate goal of understanding the governing principles of nanoparticle assembly to create multicomponent three-dimensional composite materials. Our starting point focused on the synthesis of copper(I)-oxide nanoparticles, which are known to exhibit a variety of morphologies,3 and systematically evaluated how they assemble as a function of their sizes and shapes to gain insights into their assembly characteristics. With this understanding, we were able to design methods to influence their interactions (i.e., surface functionalization, size ratios, shape control, etc.) and manipulate particle-to-particle interactions to direct the assembly process. Based on the knowledge obtained from the single-component nanoparticle assembly, we are one step closer to creating multi-component nanoparticle “molecules,” where the heterogeneity and synergy between different nanoparticles open up new possibilities for controlling material properties.
The continuous flow-synthesis platform is a novel high-throughput method for materials synthesis that can profoundly change the way nanoparticles are synthesized and assembled.4,5 The current methodology for synthesis and assembly of nanoparticles uses serial empirical exploration of reaction conditions; optimization of the process can only occur in the “next round” of experiments. A streamlined process for synthesis and assembly of nanoparticles that allows the simultaneous surveying of large reaction parameters with a multitude of reactants, templating agents, and surfactants is needed to rapidly produce and assemble nanoparticles. This is where the continuous flow-synthesis platform can contribute greatly to accelerate materials discovery. These systems can easily be designed to accommodate a “change-on-the-fly” type approach to material synthesis, where reaction conditions and parameters are dynamically tuned to control the resulting product. The exceptional precision of the system in controlling various parameters of material synthesis such as reactant concentrations, reaction time, mixing rates, and temperature, is also an added benefit compared to the limited amount of control afforded by the conventional approach to material synthesis. The continuous flow-synthesis platform also provides a method to probe multitudes of reaction parameters, significantly reducing the time and effort to explore and examine a vast number of reaction conditions and parameters. In situ characterization tools, such as absorbance, fluorescence, Raman spectroscopy, electrical, and electrochemical measurement capabilities can be integrated with the system, providing a real-time monitoring of synthesis, assembly, and nano-to-macroscopic property changes.6 External forces, such as electrical, magnetic, acoustic, and sonication fields and temperature can also be integrated into the system, enabling a higher level of control for synthesis and directed assembly of nanoparticles.
Scientific Approach and Accomplishments
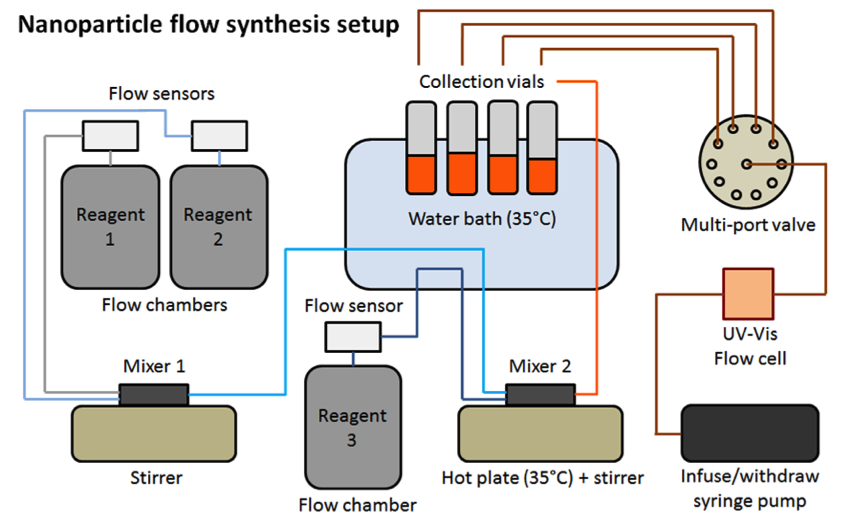
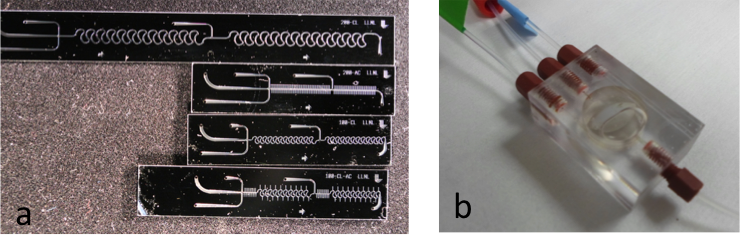
Under this project, we successfully fabricated a continuous flow-synthesis platform, shown in Figures 1 and 2. This system includes (1) an automated chemical delivery system with a precise flow-rate control of 5 µl/min to 5 ml/min, (2) an LLNL-designed micro- and macro-mixer system, (3) individually controlled heating chambers, (4) an in-situ ultraviolet-visible spectrometer, and (5) an automated sampling system. One of the advantages of these systems is their modularity. Separate “modules” can be designed, fabricated, tested, and optimized independently, which can be integrated to a single continuous flow-synthesis platform system, if needed. As seen in Figure 1, we can customize the layout of the individual components as required for different synthesis requirements. The system we designed is versatile and can adopt to multiple reaction conditions and reagents. One of the challenges in the project was designing a mixing chamber for nanomaterial synthesis. Most fluidic systems only work with solutions, however, nanomaterial synthesis requires phase separation between solids (nanomaterials) and liquids (precursors). This posed a significant challenge. In particular, synthesis of copper(I) oxide required mixing precursor solutions that resulted in the formation of copper(II) hydroxide, which is a precursor species before formation of copper(I) oxide. The formation of this particular precursor often resulted in the clogging of the micro-mixer channels. To resolve this issue, we modified the mixer design, resulting in a third-generation device shown in Figure 3(a), as well as a macromixer shown in Figure 3(b).
We have also implemented an in-situ characterization tool, namely a flow-through ultraviolet-visible spectrophotometer, which provides absorption information in a spectral range from 200 nm to 1,100 nm for the materials being studied in solution. We have determined that this technique can qualitatively distinguish nanoparticles of different sizes because of their absorbance characteristics.
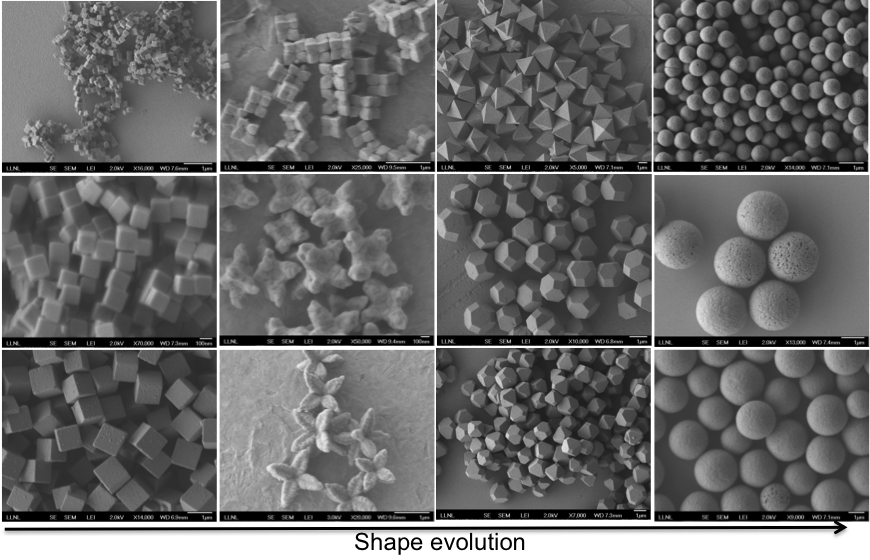
As we have stated previously, the assembly of nanoparticles with architectural complexity is still in its nascent period. However, the field of nanomaterials has advanced and matured significantly within the last decade to provide a wealth of information for both the synthesis and the functionalization of nanoparticles. We have utilized well-established protocols for both synthesis and functionalization of copper(I)-oxide nanoparticles—our model system for a shape-dependent assembly. To better understand the nuances of the nanoparticle synthesis, we also synthesized various shapes and sizes of copper(I) oxide nanoparticles, including cubes, octahedral, spheres, and flower shapes, as shown in Figure 4. We discovered that slight variations in the synthesis protocols of copper(I) oxide can dramatically change the morphology and sizes of the resulting nanoparticles, which again reaffirms the need for precise control in mixing and aging parameters during the synthesis process. This control is enabled by the continuous flow-synthesis platform system.
Impact on Mission
Our research directly supports the Laboratory’s core competency in advanced materials and manufacturing to predict the behavior of and synthesize novel materials, as well as validate the predictions. Our project also explored length scales that enhance and complement additive manufacturing efforts at LLNL. In addition, the synthesis of novel materials benefits a variety of applications in energy and climate, nonproliferation, and national security.Conclusion
We made significant advances in the design and fabrication of a new continuous flow-synthesis platform for advanced materials and successfully synthesized various shapes and sizes of nanoparticles, which demonstrated the versatility of the system. The ability to rapidly synthesize nanomaterials of various shapes and sizes will allow better understanding of shape- and size-dependent assembly of nanomaterials, bringing us closer to understanding particle assembly and to ultimately being able to predict materials assembly. The enabling technology we developed positioned Livermore to partner with the SLAC National Accelerator Laboratory in Menlo Park and Stanford University in Palo Alto, California. We are currently seeking additional support, leveraging this project for accelerating materials discovery related to the multiple-agency Materials Genome Initiative. We have also secured DOE Strategic Partnership Projects that will utilize our continuous flow-synthesis platform system.
References
- Department of Energy Workshop. Computational Materials Science and Chemistry for Innovation, Bethesda, MD, July 26–27, 2010.
- Talapan, D. V., et al., "Structural defects in periodic and quasicrystalline binary nanocrystal superlattices." JACS 133 20837 (2011).
- Huang, M. H., et al., "Synthesis of Cu2O nanocrystals from cubic to rhombic dodecahedral structures and their comparative photocatalytic activity." JACS 134, 1261 (2011).
- McFarland, E. W., and W. H. Weinberg, "Combinatorial approaches to materials discovery." Trends Biotechnol. 17, 107 (1999).
- Porter, M., Combinatorial Materials Science. Wiley-Intersciences, Hoboken, NJ (2007).
- Potyrailo, R. A., "Analytical spectroscopic tools for high-throughput screening of combinatorial materials libraries." Trends Anal. Chem. 22, 374 (2003).
Publications and Presentations
- Han, T. Y., et al., Development of rapid synthesis and characterization platform for advanced materials discovery. TechConnect World, Innovation Conf. and Expo, Washington, DC, June 15–18, 2014. LLNL-ABS-651531.