Patrick Campbell (16-LW-013)
Project Description
Because carbon dioxide (CO2) is the primary greenhouse gas emission from fossil fuel combustion, there is a need for more-efficient and less-expensive CO2 capture and sequestration. This technology needs to be inexpensive enough to economically retrofit existing power and industrial infrastructure. It needs to be applied to both power plants and industry because electricity production (38%) and industrial fossil-fuel combustion (14%) were the first and third largest sources of CO2 emissions in the U.S. for 2011, respectively. However, the installation and operational costs of CO2 capture-and-sequestration infrastructure using existing technology are staggering. An economically viable method for separating CO2 from flue gases must be developed. We plan to create a CO2 separation membrane that is sufficiently energy- and cost-efficient for retrofitting power plants and industrial combustion facilities. We are developing new capture-and-sequestration technology based upon the recent discovery of reversible CO2 adsorption by molten hydroxide electrolytes. Current amine-based CO2 separation increases fuel consumption by 25 to 40% because of the large temperature and pressure swings needed to capture and release CO2. Our molten hydroxide membrane will significantly reduce the energy required because it operates at flue gas temperatures and pressures. We intend to design a magnesium oxide aerogel support matrix to contain the molten potassium hydroxide and test the membrane under operating conditions.
We expect to apply the recent discovery of reversible CO2 solubility in molten hydroxide electrolytes to develop a new, economically viable CO2 separation technology with potential to significantly reduce CO2 emissions from large point sources. The system promises to be low-cost and energy-efficient. The major costs in traditional CO2 separation are the result of the pressure and temperature swing needed to absorb and release CO2. The molten electrolyte system will operate at flue gas temperature (300–650°C) and utilize the pressure gradient between the flue gas and atmosphere (5–20 atm). Additionally, we will use voltage gradients in a biomimetic fashion to enhance mass transport through our membranes for fast separation with low-pressure gradients. This research will also contribute to the fundamental understanding of CO2 and water adsorption in molten hydroxide systems, which could enable innovation in medium-temperature alkaline fuel cells and direct carbon fuel-cell technology, as well as provide lower energy routes to ammonia and hydrocarbon synthesis. We intend to leverage Laboratory capabilities in materials design for creating a magnesium oxide aerogel support matrix, in electrolyte development for containing molten hydroxides, and in membrane characterization for creating membranes to withstand high temperatures and pressures, which will generate intellectual property as well as high-impact publications.
Mission Relevance
By developing cost-effective technology to significantly reduce greenhouse gas emissions from large point sources such as fossil fuel power plants, this research project supports the Laboratory's strategic focus area in energy and climate security. The development of new materials and membrane technology, which have applications beyond CO2 separation in fields such as energy storage and synthetic fuels and fertilizers, is relevant to LLNL's core competency in advanced materials and manufacturing.
FY16 Accomplishments and Results
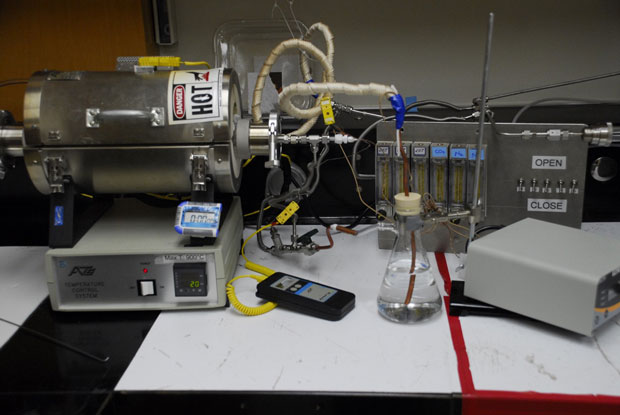
In FY16 we (1) characterized potassium hydroxide melts for CO2, water, and nitrogen solubility: argon and nitrogen were not absorbed by molten potassium hydroxide at 400°C, while 1:1 mixtures of CO2 and argon led to quantitative conversion to potassium carbonate within 20 minutes; (2) developed novel porous support matrices based on zirconium oxide, cerium oxide, and silicon carbide that are mechanically robust and stable—for zirconium oxide, we demonstrated that greater than 62 weight percent potassium hydroxide capacity is stable at 400°C for more than 192 hours over 4 heating and cooling cycles; (3) examined adhesives to mount our membranes to zirconium tubing to evaluate CO2 separation performance; and (4) added water vapor to thermogravimetric analysis and tube furnaces to measure water absorption and CO2 desorption, and began to measure CO2 separation performance (see figure).
Tube furnace with humidification and selectable gases used for testing molten salts for carbon dioxide capture.