Salmaan Baxamusa (15-ERD-020)
Project Description
Chemically stable and optically transparent polymers are broadly applicable as coatings for a variety of applications, including optical, electronic, biomedical, or sensing devices. Such materials may serve as next-generation target shells or capsule ablators used to compress the laser ignition target for Inertial Confinement Fusion experiments. Plasmas are currently used to synthesize chemical vapor-deposition polymers, but the resulting plastics are not as chemically stable or optically transparent as those synthesized by liquid-phase means. We plan to use chemical vapor deposition to synthesize polymers that are chemically stable and optically transparent. We will use non-plasma, synthetic vapor-phase strategies that mimic traditional liquid-phase polymer synthesis, thus allowing us to tailor polymer properties while maintaining the advantages of chemical vapor-deposition processing. Unlike the polymers with complex chemistry and dynamic properties from plasma chemical vapor deposition, we aim to synthesize polymers that are as simple and stable as commodity polymers made using traditional liquid-phase means. Our system will use thermal- or photo-excitation of specific chemical additives that will initiate polymerization without fragmenting the polymer precursor gas. Eliminating this fragmentation will allow us to design polymers based on predictive structure–property relationships.
We will synthesize and cast polymers that combine the processing advantages of chemical vapor deposition (conformable capability, purity, and scalability) with the tailored material properties of traditionally synthesized polymers. These chemical vapor-deposition polymers will have a fundamental chemistry that is as simple, well characterized, and stable as polymers synthesized using traditional liquid-phase means. They will thus be more chemically stable and optically transparent than current plasma chemical vapor-deposition polymers. Specifically, we intend to develop a reactor capable of creating non-plasma chemical vapor deposition of polymers from organic gases. We will develop a process for synthesizing polymers that are greater than 80% transparent over the entire visible range through a 200-µm optical slab. In addition, we will deliver a thorough general characterization (thermal, mechanical, and chemical) of a plastic synthesized from an optimized process and an enhanced understanding of the factors that govern the aging behavior of chemical vapor-deposition polymers.
Mission Relevance
Chemically stable and optically transparent chemical vapor deposition polymers would simplify target fabrication, improve ablator performance by reducing x-ray opacity, and enable detailed characterization of the critical fuel–ablator interface (an unsolved problem in target fabrication) for Inertial Confinement Fusion applications. Chemically stable and optically transparent polymers will also broadly apply to coatings for a variety of additional applications including optical, electronic, or biomedical devices. Furthermore, these polymers may be useful as surface treatments for additively manufactured components with complex topology, because it would allow for independent control of bulk and surface material properties, in support of the Laboratory's core competency in advanced materials and manufacturing.
FY16 Accomplishments and Results
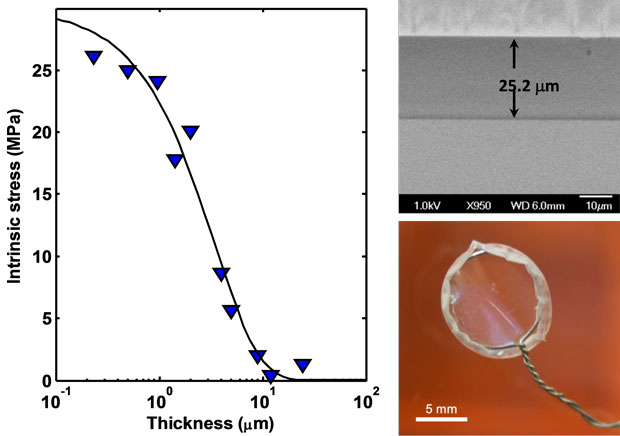
In FY16 we (1) modified our chemical vapor-deposition reactor to meet evolving needs; (2) demonstrated that poly(divinylbenzene) can be deposited as a smooth, conformal film as thick as 10 µm (see figure); (3) characterized and modified mechanical, thermal, optical, and chemical properties of poly(divinylbenzene) to meet high-level ablator requirements; (4) developed a method to measure stress in poly(divinylbenzene) films; (5) began designing a stage for coating spherical substrates; and (6) developed ultrathin adhesive films for fabricating novel ablator assemblies.
Publications and Presentations
- Baxamusa, S., et al., Nuclear fusion, microbonding, and more: HWCVD polymers at Lawrence Livermore. 9th Intl. Conf. on Hot Wire (Cat) and Initiated Chemical Vapor Deposition, Philadelphia, PA, Sept. 6–9, 2016. LLNL-ABS-691082.
- Baxamusa, S., et al., “Photo-oxidation of polymer-like amorphous hydrogenated carbon under visible light illumination.” Polymer Degrad. Stabil. 122, 133 (2016). LLNL-JRNL-677678.
- Baxamusa, S., et al., “Photo-oxidation of polymers synthesized by plasma and initiated chemical vapor deposition.” Chem. Vapor Deposit. 21, 267 (2016). LLNL-JRNL-669872.
- Baxamusa, S., et al., Photo-oxidation under visible light: the strange case of plasma polymers, Service Life Prediction of Polymeric Materials: Over the Horizon, Santa Fe, NM, Mar. 20–24, 2016. LLNL-ABS-678481.
- Lepro, X., et al., Measuring the thermal stress and stability of poly (divinylbenzene) films produced by HWCVD. 9th Intl. Conf. on Hot Wire (Cat) and Initiated Chemical Vapor Deposition, Philadelphia, PA, Sept. 6–9, 2016. LLNL-ABS-691088.