Thomas P. Guilderson (17-ERD-061)
Abstract
We have generated a proof-of-principal 129I dataset from the subtropical North Pacific demonstrating its conventional transient tracer utility. 129I and 3H (tritium) have very similar density–concentration profiles, including the transition from well-mixed, near-constant concentration to non-perturbed tritium and deep water free of weapons-derived 129I. This tracer front is deeper than that of chlorofluorocarbon (CFCs) and sodium hexafluoride (SF6) and reflects the differing time-histories of the input of these tracers into the surface ocean. The similarity between the 3H and 129I front implies that, regardless of the different processes influencing tritium and iodine, the two tracers could be used together to better define the age and age-distribution of waters that were last at the surface of the ocean between 1957 and 1963.
Exploration of North Pacific Subtropical Gyre (NPSG) ocean-surface ∆14C with a 1-dimensional box diffusion model provided insight into the local vertical diffusivities between the well-mixed ocean surface and the interior ocean. The implied diffusivity, approximately 0.6 cm2-sec-1, is lower than that of the global ocean.
Background and Research Objectives
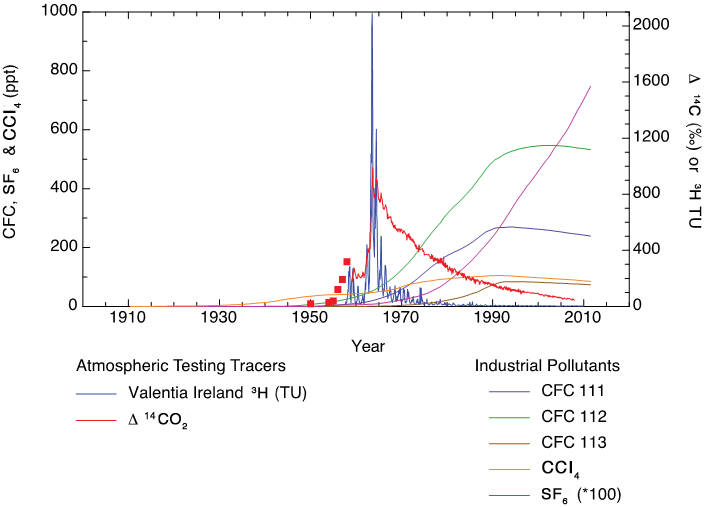
Transient tracers (i.e., chemical tracers with time-varying sources or sinks) such as 3H, CFCs, SF6, and “bomb-derived” radiocarbon (14C) (see Figure 1) have been used to elucidate the pathways and timescale of ocean ventilation and mixing (e.g., Bullister and Weiss 1983; Fine et al. 1987; Key et al. 2002; Jenkins 1988; Stuiver and Östlund, 1980; Warner et al. 1996; Weiss et al. 1979). A better understanding of the mechanisms and prediction of the uptake and redistribution of anthropogenic carbon dioxide (CO2) and heat by the ocean are key to determining how that process works. Transient tracers have been used as diagnostic tools that provide insights about the effectiveness of ocean models (Dutay et al. 2002; Graven et al. 2012a; Guilderson et al. 2000; Orr et al. 2002; Matsumoto et al. 2004), as well as for determining anthropogenic-carbon uptake by the ocean (Hall et al. 2002; McNeil et al. 2003; Sonnerup et al. 2008).
Small changes in water-mass ages may have a significant effect on the implied rate of CO2 uptake and distribution of anthropogenic CO2 (Sonnerup et al, 2008). The implied rate of oceanic (net) anthropogenic-CO2 uptake cascades into fundamental uncertainty in the instantaneous/annual partitioning of fossil-fuel CO2 emissions between the atmosphere, ocean, and terrestrial biosphere reservoirs. Of the three reservoirs, the atmosphere is quantitatively the best known, whereas oceanic uptake is estimated via empirical relationships, ocean general circulation models (OGCMs), and tracer-based inversions (e.g., Orr et al. 2002; Le Quéré et al. 2009; Hall et al. 2002; and Khatiwala et al. 2009, 2013). The terrestrial biosphere is the least constrained of the three reservoirs; its CO2 uptake is determined by the difference between what is measured in the atmosphere and modeled for the ocean. The time-histories of the ocean's uptake of anthropogenic CO2 derived from tracer-based inversions and OGCMs are not necessarily consistent with each other and may lead to divergent views on the efficacy and magnitude of the terrestrial carbon sink (Khatiwala et al, 2013). In the case of the uptake and redistribution of heat (i.e., ocean heat content), it is thought that problems with decadal climate predictions, including natural multi-decade variability, are directly tied to model biases or inaccuracies of the uptake and redistribution of heat. This may include the failure of some coupled climate models to adequately predict the rise of global temperatures observed after 1998 (e.g., Steinman et al, 2015; He and Soden, 2016).
Observations and modeling can be used to diagnose model biases or to place observations in a dynamic context that can better elucidate forcing mechanisms. In previous work using what were once state-of-the-art ocean models, we almost always came up with a data-model mismatch for the timing and amplitude of the post-bomb 14C peak, regardless of whether the model used was a large-scale OGCM or a higher-resolution regional model with a focus on the Pacific. The models were accumulating too much anthropogenic (bomb) 14C too quickly. It was thought that this "feature" reflected a general failing of adequate exchange between the ocean's surface mixed layer (well-mixed due to surface winds) and the interior buoyancy-driven circulation. The matches were poor even for the subtropical regions where air–sea CO2 exchange (a factor that we know reasonably well) should dominate the signal (i.e., where the models should have performed better).
Focusing on the NPSG, we proposed (1) developing 129I as an additional tracer for determining the timescale of interior ventilation, and (2) exploring the ocean surface response of anthropogenic radiocarbon, with a focus on coupling between the ocean's surface mixed layer and its deeper interior circulation.
Scientific Approach and Accomplishments
Inorganic Iodine: An Underexploited Oceanographic Transient TracerInorganic iodine is a micronutrient that is nearly conservative in open-ocean seawater, exhibiting only a weak depletion in surface waters relative to the deep ocean (Barkley and Thompson, 1960; Elderfield and Truesdale, 1980; Nakayama et al, 1989). In the open ocean, total inorganic-iodine concentrations are approximately 0.47 micromoles and total iodine is speciated between iodate (IO3-) and iodide (I-). Iodate–iodide partitioning in most of the ocean is relegated to the surface mixed layer and/or the euphotic zone (i.e., the uppermost 200-250 meters). The majority of total inorganic iodine is as iodate, particularly at depth. The residence time of iodine in the ocean is approximately 340,000 years (Broecker and Peng, 1982).
Isotopes of iodine include several short-lived isotopes (e.g., 131I, half-life of eight days), one stable isotope (127I), and 129I, a long-lived (half-life of 15.7 x106 yrs) radioisotope. The 129I radioisotope is naturally produced in the atmosphere via cosmic-ray interactions with atmospheric xenon and as a fission product of uranium. The relative production of these two processes is thought to be about equal (Fabryka-Martin et al, 1987). The low production of 129I combined with a long oceanic residence time to yield a uniform 129I content (129I/127I) in seawater of ≤1.5 x 10-12 (Moran et al, 1995). Anthropogenic 129I has been produced and dispersed via atmospheric testing of nuclear weapons (90kg ±50%) and more recently via nuclear-fuel reprocessing, with most of the reprocessing byproducts being discharged into the North Atlantic (Snyder et al, 2010).
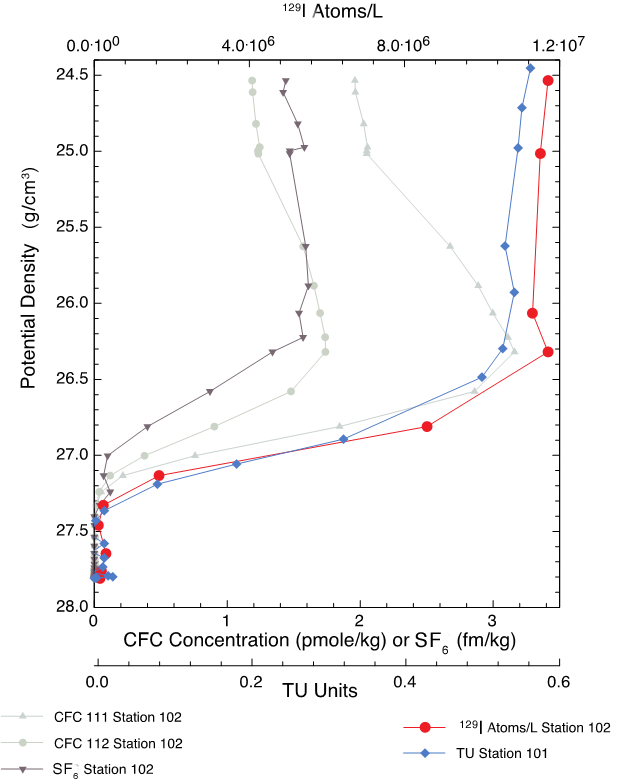
We collected a proof-of-principle dataset of subtropical North Pacific total inorganic 129I (Figure 2) that demonstrates the utility of employing 129I derived from weapons testing as an oceanographic tracer. Because of its 15.7-million-year half-life, 129I is (unlike shorter-lived isotopes such as 3H with its 12-year half-life) a permanent dye-tracer. The initial input function of anthropogenic 129I for the North Pacific (determined from rainfall measurements) exceeds four orders of magnitude and (unlike CFCs and SF6) is a simple step function. Thus, 129I carries information that is different from (but complementary to) the existing and commonly applied transient-tracer suite.
Figure 2. CFC concentration versus potential density (in grams per cubic centimeter, g/cm3), a proxy for depth (but more consistent with how water moves in the deep ocean) of industrial pollutants CFC111, CFC112, SF6 (all from Climate Variability and Predictability [CLIVAR] Map Section P02 Station 102, 152°W, 30°N), as well as 129I from Station 102, and tritium (3H) from Station 101 (153°W, 30°N).
As shown in Figure 2, 129I and 3H have very similar density–concentration profiles, including the transition from well-mixed, near-constant concentration to non-perturbed 3H and weapons-derived 129I-free deep water. This tracer front is deeper than that of CFCs/SF6 and reflects the differing time-histories of the input of these tracers into the ocean surface. The similarity between the 3H and 129I fronts implies that, regardless of the different processes influencing 3H and iodine content, the two tracers could be used together to better define the age and age-distribution of waters that were last at the surface of the ocean between 1957 and 1963, the height of the atmospheric testing of nuclear weapons.
Air–Sea 14CO2 ExchangeThe time history of the ocean's uptake and redistribution of bomb-derived 14CO2 is a diagnostic of local and large-scale processes. The post-bomb rise in surface ∆14C is a unique diagnostic of air–sea CO2 exchange (Mahdevan 2001; Guilderson et al, 2000); the uptake of bomb-derived 14C has been used to infer air–sea CO2 exchange rates (Broecker and Peng 1982; Sweeney et al, 2007; Wannikhof et al, 1992). We compared the response of the ocean to atmospheric ∆14C in a 1-dimensional box-diffusion model with reconstructions of the NPSG.
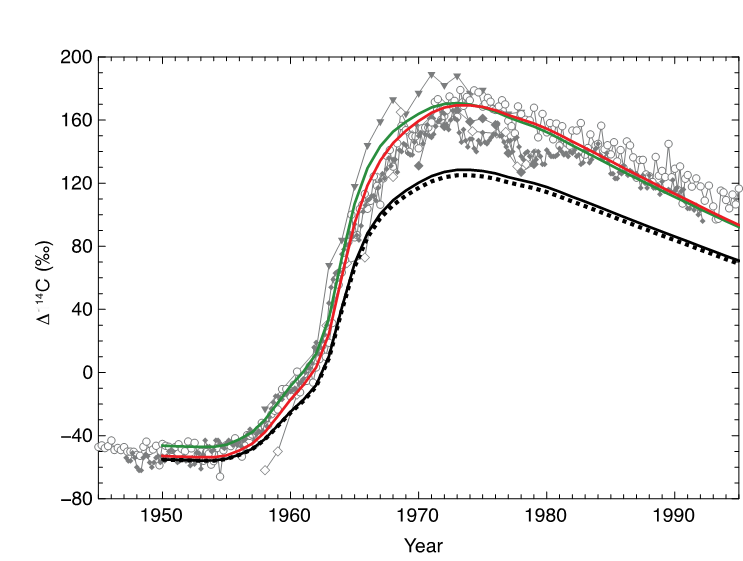
The box-diffusion model used (Figure 3) is based on that of Oeschger et al. (1975) and used by Stuiver et al (1983, 1986). This model has two parameters that, over longer time periods, can play off of each other: air-sea CO2 exchange (G, moles-m-2-yr-1) and ocean vertical diffusivity (Kz, cm2-sec-1). A constraint on the long-term integration of G and Kz is the average ∆14C value of the deep-ocean (-190 per mil: Östlund and Stuiver, 1980). For our sensitivity experiments, the box-diffusion model was initialized in 1500 AD using reconstructed atmospheric ∆14C (Reimer et al, 2013) and allowed to spin-up for 1,000 years. It was then run forward to 1950 AD. In 1950 it was then forced with the mid-latitude northern hemisphere 14C composite of Hua et al. (2013). The manually-derived best-fit to the NPSG ocean surface reconstructions (Figure 3), while meeting the deep Pacific ∆14C requirement, requires a ~30 percent higher gas exchange (25 vs 19 mole-m-2-yr-1) relative to the reference curve and a Kz that is increased by ~10 percent (1.4 vs 1.26 cm-2-s-1). A similarly high gas-exchange rate is required to capture the post-bomb reconstructed surface water ∆14C history of the Sargasso Sea (not shown).
The required local G, and its corollary k, the gas transfer, is higher than that inferred from a global ocean inventory of bomb-derived14C (e.g., Sweeney et al, 2007; Wannikhof 2009) and similar 1-dimensional estimates for other surface-14C reconstructions (e.g., Chakraborty et al, 1994; Fallon et al, 2003). Reconstructed winds for Hawaii (DaSilva et al, 1994) are not significantly different during the rise in atmospheric 14C relative to the climatological mean: temporally higher velocity winds, in addition to increasing bubble injection and breaking waves, would result in better air-sea exchange of the bomb transient. We interpret our high-G requirement to be a consequence of attempting to reconstruct the regional response of surface ∆14C, where only shallow, potential densities approaching 25.0 sigma_t, are ventilated. If this interpretation is correct, and under this presumption, we can relax the constraint on deep Pacific radiocarbon values while keeping G fixed at 19 mole-m-2-yr-1, an air-sea flux more consistent with the local wind speed. A good fit to the coral-based ∆14C reconstructions is with a Kz= 0.62 cm2-s-1 (Figure 3). Although released from the constraint of deep Pacific ∆14C, this Kz provides a reasonable fit to pre-bomb observations of ∆14C (-100 per mil) in deep-sea corals from the NPSG at the top of the mesopelagic 400-500m water depth (Roark et al, 2006). The inference is that the Kz for the upper subtropical Pacific is lower than that estimated for the whole of the ocean.
Impact on Mission
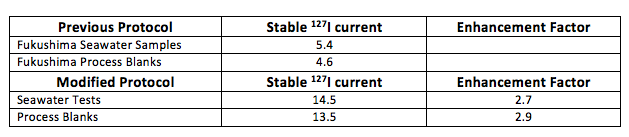
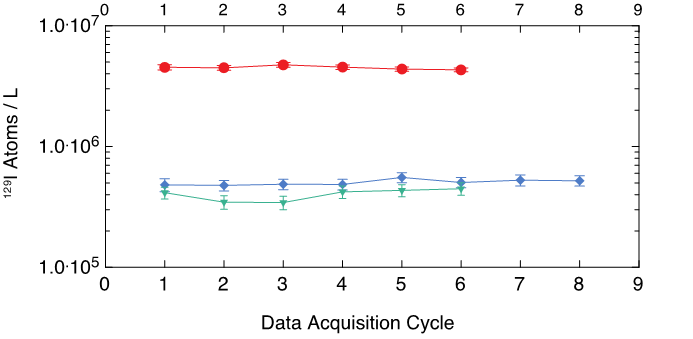
We used a methods-development aspect as part of the 129I analyses. The end result was a nearly three-fold increase in ion current (Table 1) out of the target matrix (relative to data) that we generated to document the Fukushima Nuclear Power Plant catastrophe (Guilderson et al, 2013; Tumey et al, 2013).
Both protocols utilized 250ml field samples (approximately 15 micrograms of iodine) and approximately 500 milligrams of carrier. Both protocols yielded the same concentration on common materials. The current increase can be explained by a nearly two-fold increase in recovery and an increase in sputtering efficiency of the target matrix. The increased currents will enable more precision per unit time on target (Figure 4) and allow for smaller field samples. Future oceanographic tracer data will be uniformly consistent with the uncertainty of CFC, SF6 and 3H data.
The sensitivity improvement of environmental 129I measurements produced by this project has enhanced the utility of 129I as a conventional transient tracer, which is of interest to sponsors such as the NNSA. 129I is a major fission fragment and is released to the environment during nuclear-fuel reprocessing, which makes it a powerful signature of clandestine activity.
Conclusion
In addition to the already submitted manuscript, we anticipate submitting at least two additional manuscripts. The first is the 129I proof-of-principal dataset, and the second is an NPSG comparison with tracer enabled OGCMs. The OGCM study is providing an entry way into an international Ocean Modeling Intercomparison Project, which is being undertaken under the Coupled Model Intercomparison Project Phase 6.
References
Andrews, A.H., et al. 2016. "Bomb Radiocarbon and the Hawaiian Archipelago: Coral, Otoliths, and Seawater." Radiocarbon 58, 531-548.
Barkley, R.A., and T.G. Thompson. 1960. "The Total Iodine and Iodate-Iodine Content of Seawater." Deep-Sea Research 7, 24-34.
Broecker, W.S., and T.H. Peng. 1982. "Tracers in the Sea." ELDIGIO Press, Palisades, NY.
Bullister, J.L., and R.F. Weiss. 1983. "Anthropogenic Chlorofluorocarbons in the Greenland and Norwegian Seas." Science 22, 265-268.
Chakraborty, et al. 1994. "Air-Sea Exchange of CO2 in the Gulf of Kutch, Northern Arabian Sea Based on Bomb-Carbon in Corals and Tree Rings." Proceedings Indian Academy of Sciences 103, 329-340.
DaSilva, A., et al. 1994. "Atlas of Surface Marine Data 1994, a Five-Volume Atlas Series Depicting the Seasonal and Yearly Variations of the Surface Marine Atmosphere over the Global Oceans." National Oceanic and Atmospheric Administration, Washington, DC.
Druffel, E.R.M. 1987. "Bomb Radiocarbon in the Pacific: Annual and Seasonal Timescale Variations." Journal of Marine Research 45, 667-698.
Druffel, E.R.M., et al. 2001. "Changes in Subtropical North Pacific Radiocarbon and Their Correlation with Climate Variability." Radiocarbon 43, 15-25.
Dutay, J-C., et al. 2002. "Evaluation of Ocean Model Ventilation with CFC-11: Comparison of 13 Global Ocean Models." Ocean Modelling 4, 89-120.
Elderfield, H., and V.W. Truesdale. 1980. "On the Biophilic Nature of Iodine in Seawater." Earth and Planetary Science Letters 50, 105-114.
Fallon, S.J., et al. 2003. "Carbon Isotope Constraints on Vertical Mixing and Air-Sea CO2 Exchange." Geophysical Research Letters 30. doi:10.1029/2003GL018049.
Fabryka-Martin, J., et al. 1987. "Applications of 129I and 36Cl in Hydrogeology." Nuclear Instruments and Methods in Physics Research B29, 361-371.
Fine, R.A., et al. 1987. "The Penetration of Tritium into the Tropical Pacific." Journal of Physical Oceanography 17, 553-564.
Graven, H.D., et al. 2012a. "Changing controls on Oceanic Radiocarbon: New Insights on Shallow-to-Deep Ocean Exchange and Anthropogenic CO2 Uptake." Journal of Geophysical Research 117. doi:10.1029/2012JC008074.
——— 2012b. "Observations of ∆14C in CO2 at La Jolla, California, 1992-2007: Analysis of the Long-Term Trend." Journal of Geophysical Research: Atmospheres 117, D02302. doi:10.1029/2011JD016533.
Guilderson, T.P., et al. 2000. "Radiocarbon as a Diagnostic Tracer in Ocean and Carbon Cycle Modeling." Global Biogeochemical Cycles 14, 887-902.
——— 2014. "The 129Iodine Content of Subtropical Pacific Waters: Impact of Fukushima and Other Anthropogenic 129I Sources." Biogeosciences 11, 4839-4852. doi:10.5194/bg-11-4839-2014.
Hall, T.M., et al. 2002. "Inferring the Concentration of Anthropogenic Carbon in the Ocean from Tracers." Global Biogeochemical Cycles 16, 1131.
He, J., and B.J. Soden. 2016. "The Impact of SST Biases on Projections of Anthropogenic Climate Change: A Greater Role for Atmosphere-Only Models?" Geophysical Research Letters 43, 7745-7750. doi:10.1002/2016GL069803.
Hua, Q., et al. 2013. "Atmospheric Radiocarbon for the Period 1950-2010." Radiocarbon 56, 2,059-2,072.
Jenkins, W.J. 1988. "The Use of Anthropogenic Tritium and Helium-3 to Study Subtropical Gyre Ventilation and Circulation." Philosophical Transactions of the Royal Society of London, A., 325, 1583, 43-59.
Key, R. M., et al. 2002. "WOCE Radiocarbon IV: Pacific Results." Radiocarbon 44, 239-392.
Khatiwala, S., et al. 2009. "Reconstruction of the History of Anthropogenic CO2 Concentrations in the Ocean." Nature 462, 346-349.
——— 2013. "Global Ocean Storage of Anthropogenic Carbon." Biogeosciences 10, 2169-2191.doi:10.5194/bg-10-2169-2013.
Levin, I., and B. Kromer. 2004. "The Tropospheric 14CO2 Level in Mid-latitudes of the Northern Hemisphere (1959-2003)." Radiocarbon 46, 1,261-1,272.
Le Quéré, C., et al. 2010. "Impact of Climate Change and Variability on the Global Oceanic Sink of CO2." Global Biogeochemical Cycles 24, GB4007.
Mahdavan, A. 2001. "An Analysis of Bomb Radiocarbon Trends in the Pacific." Marine Chemistry 73, 273-290. doi:10.1016/S0304-4203(00)00113-4.
Matsumoto, K., et al. 2004. "Evaluation of Ocean Carbon Cycle Models with Data-Based Metrics." Geophysical Research Letters 31, L07303. doi:10.1029/2003GL018970.
McNeil, B.I., et al. 2003. "Anthropogenic CO2 Uptake by the Ocean Based on the Global Chlorofluorocarbon Dataset." Science 299, 235-239.
Moran, J.E., et al. 1995. "Determination of Source Ages and Migration Patterns of Brines from the U.S. Gulf Coast Basin Using 129I." Geochimica et Cosmochimica Acta 59, 5055–5069.
Nakayama, E., et al. 1989. "Chemical Analyses of Seawater for Trace Elements. Recent Progress in Japan on Clean Sampling and Chemical Speciation of Trace Elements, a Review." Analytical Sciences 5, 129-139E.
Oeschger, H., U., et al. 1975. "A Box Diffusion Model to Study Carbon Dioxide Exchange in Nature." Tellus B 27, 168-192.
Östlund, H.G., and M. Stuiver. 1980. "GEOSECS Pacific Radiocarbon." Radiocarbon 22, 25-53.
Orr, J.C. 2002. "Global Ocean Storage of Anthropogenic Carbon (GOSAC), Final Report." EC Environment and Climate Programme, 117pp.
Reimer, P.J., et al. 2013. "Intcal 13 and Marine13 Radiocarbon Age Calibration Curves 0-50,000 Years Cal BP." Radiocarbon 55, 1,869-1,887.
Roark, E.B., et al. 2006. "Radiocarbon Based Ages and Growth Rates: Hawaiian Deep-Sea Corals." Marine Ecology Progress Series 327, 1-14.
Snyder, G., A. et al. 2010. "Global Distribution and Long-Term Fate of Anthropogenic 129I in Marine and Surface Water Reservoirs." Geochemistry, Geophysics and Geosystems 11, 4. doi:10.1029/2009GC002910.
Sonnerup, R.E., et al. 2008. "Improved Estimates of Ventilation Rate Changes and CO2 Uptake in the Pacific Ocean Using Chlorofluorocarbons and Sulfur Hexafluoride." Journal of Geophysical Research 113, C12007.
Steinman, B.A., et al. 2015. "Atlantic and Pacific Multidecadal Oscillations and Northern Hemisphere Temperatures." Science 347, 988-991.
Stuiver, M., et al. 1983. "Abyssal Water Carbon-14 Distribution and the Age of the World Oceans." Science 18, 849-851.
Stuiver, M., et al. 1986. "Radiocarbon Age Calibration of Marine Samples Back to 9000 cal yr BP." Radiocarbon 28, 980-1021.
Sweeney, C., et al. 2007. "Constraining Global Air-Sea Gas Exchange for CO2 with Recent Bomb 14C Measurements." Global Biogeochemical Cycles 21, GB2015. doi:10/1029/2006GB002784.
Toggweiler, J.R., et al. 1991. "The Peru Upwelling and the Ventilation of the South Pacific Thermocline." Journal of Geophysical Research-Oceans 96, 20,467-20,497.
Tumey, S.J., et al. 2013. "Input of 129-I into the Western Pacific Ocean Resulting from the Fukushima Nuclear Event." Methods and Applications of Radioanalytical Chemistry 296, 2, 957-962. doi:10.1007/s10967-012-2217-9.
Wanninkhof, R. 1992. "Relationship Between Gas Exchange and Wind Speed over the Ocean." Journal of Geophysical Research-Oceans 97, 7373-7381.
Wanninkhof, R., et al. 2009. "Advances in Quantifying Air-Sea Gas Exchange and Environmental Forcing." Annual Reviews in Marine Science 1, 213-244.
Warner, M.J., et al, 1996. "Basin-Wide Distributions of Chlorofluorocarbons CFC-11 and CFC-12 in the North Pacific: 1985-1989." Journal of Geophysical Research 101, 20525-20542.
Weiss, W., et al. 1979. "Evidence of Pulsed Discharges of Tritium from Nuclear Energy Installations in Central European Precipitation." Behavior of Tritium in the Environment Proceedings Series, pp. 17-30, IAEA-SM-232/18, IAEA, Vienna.
Publications and Presentations
Guilderson, T. P., et al. 2017. "Post-Bomb Subtropical North Pacific Surface Water Radiocarbon History." Submitted/in-review Oceanography, LLNL-JRNL-740486.