Michael Bagge-Hansen (15-LW-074)
Abstract
Nanoporous metals have attracted significant attention for applications in catalysis, sensors, and optical devices due to their high specific surface area, low density, continuous conductive structure, and novel nanoscale properties. We developed a facile method to produce nanoporous metal powders by freeze-drying aerosolized aqueous metal-salt solutions and subsequent thermal treatment. Several grams of nanoporous metal powder can be prepared using our bench-top setup, which offers straightforward strategies to scale-up production. This capability may provide an economically viable method to produce nanoporous metal micro-particles with extremely high yield and purity. We selected nanoporous copper and silver to demonstrate the technique, though other nanoporous metals may be produced using the same method. The structure and morphology of nanoporous metal products are determined by complex processes that occur during freezing, drying, and thermal treatment. We systematically investigated these fundamental processes using a combination of in situ and ex situ characterization during and after freeze-drying, including x-ray diffraction, ultra-small-angle x-ray scattering (USAXS), and electron microscopy. In particular, the application and development of in situ USAXS for freeze-drying provided new insights into freeze-drying phenomena, including dramatic changes in the ligament morphology during drying. These new synthesis and characterization capabilities form the basis for continued interest in freeze-drying science for the development of novel materials at Lawrence Livermore National Laboratory.
Background and Research Objectives
Nanoporous materials are three-dimensional self-supporting structures that resemble foams. The interconnected ligaments and struts (typically less than 500 nm in diameter) form complex networks that provide continuous, open porosity that is greater than 50 percent (Tappan et al. 2010). This nano-architecture is characterized by very high specific surface areas and low density. These materials often display surprising electronic, chemical, or mechanical properties that are substantially different from their bulk counterparts. While nanoporous compounds such as silica and alumina are mass-produced (an expensive process), nanoporous metals have conventionally required much more complex synthesis strategies that have been a barrier to broader use in processes such as templated assembly, dealloying, sol-gel processes, nanosmelting, and combustion synthesis. Nonetheless, nanoporous metals are much sought after for use in a large number of applications such as catalysis, battery and capacitor electrodes, heat sinks, hydrogen storage, filtration, and antimicrobial scaffolds (Tappan et al. 2010). At Lawrence Livermore National Laboratory, nanoporous metals are needed for independent control of material composition and density in high-energy physics experiments, battery and sensor materials fabrication, and basic science experiments investigating the mechanical and chemical properties of these materials. In many of these applications, powdered nanoporous material offer distinct advantages, including improved homogeneity, mass transport, shape control, and process integration.
The goals of this project were to (1) develop nanoporous copper and silver metal powders by leveraging our initial observation that nanoporous salts could be formed from the rapid freezing of aerosolized solutions and possibly converted to metal through subsequent thermal or chemical treatments; (2) demonstrate techniques for tuning the properties of nanoporous metal powders, including density and particle size; and (3) develop and apply advanced in situ characterization approaches to study all aspects of freeze-drying synthesis, with particular emphasis on synchrotron-based ultra-small-angle x-ray scattering (USAXS). We have achieved all of these project goals and the team is finalizing at least two publications describing the results of our research. Additionally, the significant intellectual property resulting from our work is the subject of a patent application filed by the project team. The technologies and fundamental science developed for this project form an important foundation from which new initiatives and directions for advanced materials research and development have grown.
Scientific Approach and Accomplishments
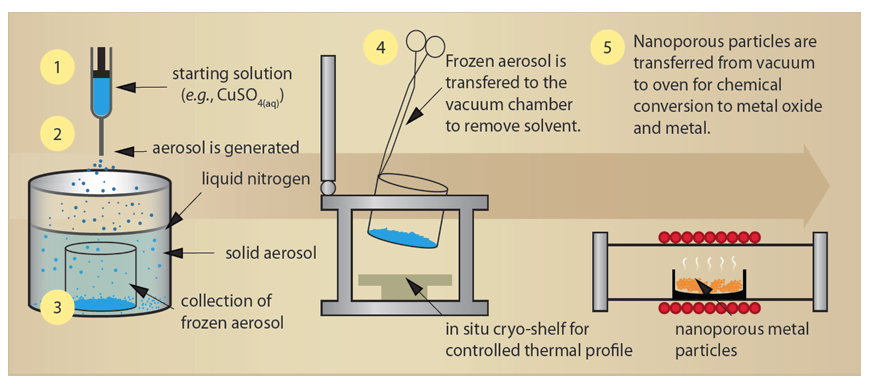
The primary objective of this project was the development of nanoporous copper and silver metal powders from our preliminary observation that nanostructured salt could be produced by the rapid freezing and subsequent drying of aerosols. The process of freeze-drying of aerosols requires five essential steps (Figure 1): (1) formulating an amenable solution; (2) aerosolizing the solution; (3) cryogenically freezing the aerosol; (4) collecting and vacuum desiccating the frozen aerosol; and (5) conversion of the resulting nanoporous particles to the desired chemical composition, typically with controlled-environment thermal processing (e.g., reduction of copper sulfate to metallic copper).
These processes must be controlled in such a way that the nanoporous structure is preserved. For example, we found that the final product’s mechanical stability, density, and porosity are very sensitive to the starting solute's concentration, the geometry of freezing, and the thermal profile during vacuum desiccation. While the process appears to be straightforward, the successful and repeatable production of nanoporous metals required extensive development. First, a highly controlled aerosol-delivery method using an ultrasonic nozzle (20—180 kHz) was implemented. The size distribution of the generated aerosol can then be prescribed by selection of the driving frequency. During these experiments, the mean droplet-diameter distribution could be selected from 10—50 µm. The consistency and size of the aerosol droplets also enables prompt and uniform freezing when the aerosol comes in contact with the cryogenic liquid (e.g., liquid nitrogen). Once frozen, various methods were explored for capturing, removing, and transferring the product to the vacuum chamber for drying. A nested borosilicate beaker proved most reliable, when used with a small amount of residual cryogen to prevent convective heating of the frozen powder. A specialized vacuum-drying set-up was also developed to provide a controlled temperature shelf, product-temperature feedback, and gas-independent pressure tracking. These efforts, used in concert, proved very successful: over the course of this project, we have scaled single batch production from a few milligrams to several grams while dramatically improving the end product's homogeneity and the repeatability of the process.
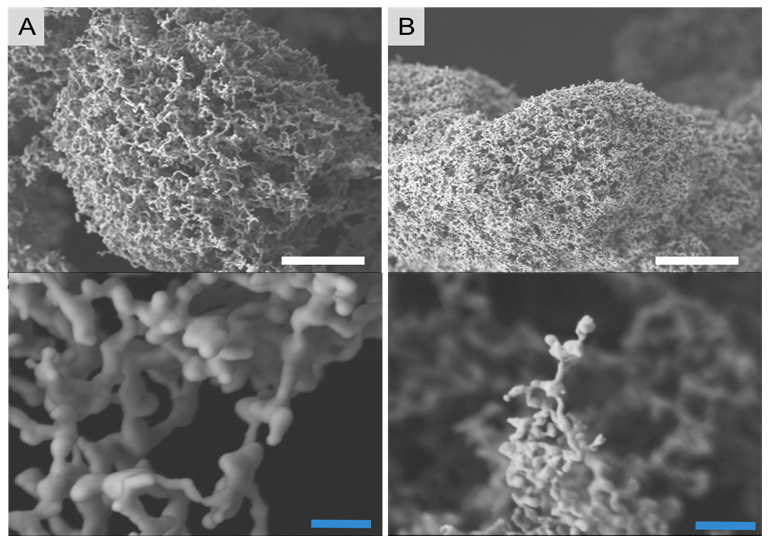
In parallel with process development, we systematically explored a variety of aqueous metal-salt solutions as feedstock to produce nanoporous metal powders. The common requirements we developed to down-select metal salts were (1) moderate solubility in water, (2) low handling hazard, and (3) a thermochemical technique for decomposing the salt to pure metal. Ultimately, we found that copper foams are best generated from copper sulfate (Figure 2A).
Notably, these powders retained the sphericity and narrow size distribution of the feedstock aerosol. Nanoporous copper-sulfate powders can be thermally converted to nanoporous copper-oxide powders by heating them to approximately 600°C. A second thermochemical treatment is required to further reduce the copper oxide to metallic copper; this can be accomplished at approximately 250°C in hydrogen gas. The resulting nanoporous copper structure has an open porosity and ligaments approximately 200 nm in diameter. Higher-temperature treatments led to coarsening of the ligament structure. In some cases we were able to measure a small amount of residual sulfur. Catalytic testing of these copper-based materials confirmed that residual sulfur can have a detrimental effect on the activity of these materials.
Our efforts to produce pure silver foams were also very successful. Silver foams are easily produced from silver acetate (Figure 2B). The discovery of the viability of the production of nanoporous silver-acetate foams was a significant achievement for this project. While many of the metal salts investigated are strongly hygroscopic (including copper sulfate), we found that silver-acetate foams are surprisingly air-stable; further, the thermal conversion of silver acetate to pure metallic silver can be done in a single step at a relatively low temperature (approximately 180°C). As a result, the size of physical features in silver foams was substantially smaller than that of copper foams—in many cases less than 50 nm. Because of the stability of these materials, we focused our advanced characterization efforts on nanoporous silver-acetate powder and the thermal conversion to nanoporous silver. We employed x-ray photoemission spectroscopy and x-ray diffraction to confirm that the resulting nanoporous structures were pure metallic silver. Furthermore, because of its importance to the end-use of these materials, we focused our experimental campaign on the changing morphology of silver-acetate foams during both freeze-drying and thermal conversion to metallic silver, and subsequently to leverage this knowledge to prevent end-product densification and optimize the product.
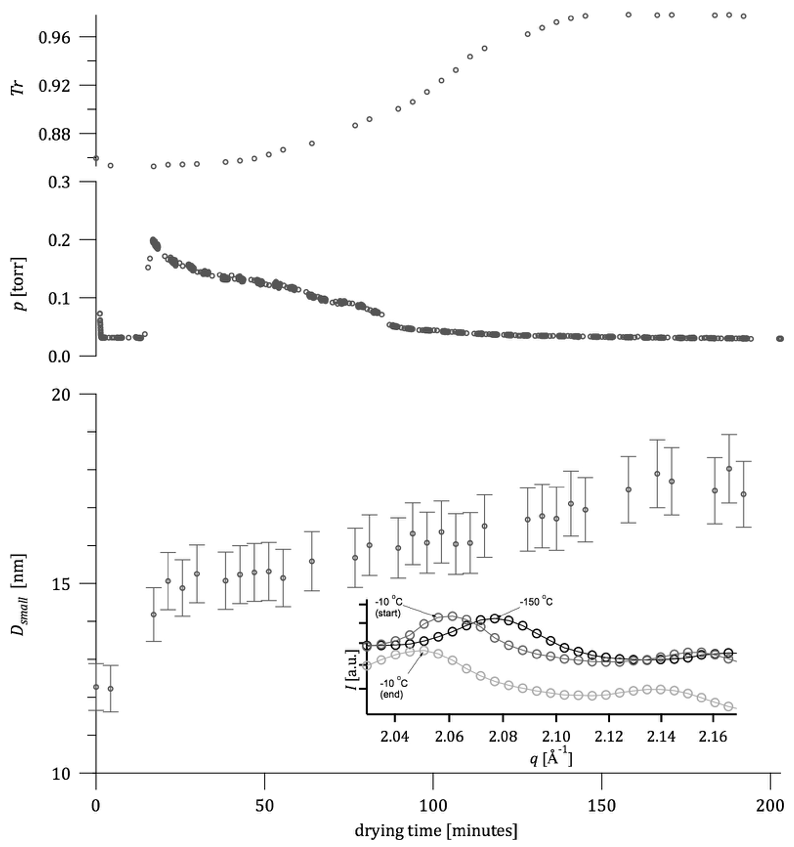
We conducted a series of in situ x-ray scattering experiments (see Figure 3) at beamline 9IDC at the Advanced Photon Source, Argonne National Laboratory (Ilavsky et al. 2013). These are the first in situ studies of freeze-drying phenomena in these materials using this approach.
The technique permits time-resolved study of ligament size and morphology from the atomic to micron scale from the as-frozen state through to the fully dry state. To accomplish this, cryogenically frozen silver acetate aerosols were prepared adjacent to the beamline and packed into a custom-designed sample cell. The sample cell was then inserted into a pre-cooled (-150°C) Linkam THMS350V temperature/pressure-controlled stage that was subsequently sealed and evacuated using a vacuum pump. The pressure in the Linkam stage was recorded in real time as the frozen aerosols dried by slow sublimation of the ice at -10°C over a period of several hours. Lower temperatures (as low as -40°C) were also explored with very minor differences in the USAXS evolution. To observe changes in the foam morphology during drying, time-resolved x-ray scattering data was collected every 5 to 10 minutes over an extremely broad q-range (0.0001 Å < q < 6.3 Å), which translates to features in the micron-size to atomic-size scale (Figure 3). No beam damage was observed in the USAXS. The changing transmission through the cell was also used as an internal monitor of the rate of ice sublimation (Figure 3). From our analysis, using appropriate models and software (Ilavsky and Jemian 2009, Glatter and Kratky 1982), we were able to extract the changing morphology of silver-acetate ligaments from as-frozen aerosol, to fully-dry nanoporous powder. Interestingly, we observed significant, systematic, and unexpected changes during the drying period that are not easily reconciled with existing freeze-drying models. Importantly, the approach we have demonstrated is relevant (and easily extended) to a wide range of materials and process parameters to enable direct observation of the underlying morphology, especially during the poorly understood early sublimation stages in freeze-dried materials.
Impact on Mission
This project supports Lawrence Livermore National Laboratory’s capabilities in nanoporous materials synthesis, as well as advanced in situ materials characterization, in support of the core competency in advanced materials and manufacturing. The specific development of low-density metal foams by a new scalable approach has relevance to materials for energy conversion, storage, and harvesting that support the Laboratory's energy- and climate-security missions. Furthermore, the synthesis of low-density metals and fundamental advances in the understanding of freeze-drying through in situ materials characterization are important to the production of materials for laser experiments to explore high-energy-density physics in support of the nuclear weapons and stockpile stewardship mission. Freeze-drying and freeze-casting techniques are increasingly incorporated into the synthesis and development of materials for programmatic impact.
Conclusion
We have developed a new approach to the synthesis of nanoporous copper and silver by freeze-drying aerosols of aqueous metal-salt solutions and subsequent thermal treatment. The scalability of this approach offers distinct advantages for future industrial applications of nanoporous materials. Our research has also shown that this process could be a paradigm for the production of new porous materials. We demonstrated the capability to study fundamental aspects of freeze-drying through in situ USAXS. We are also extending these techniques more broadly to other material systems where freeze-drying or freeze-casting are used during the respective synthesis processes. Our research has successfully established new freeze-drying science capabilities that have a promising and active role in future materials development in support of the Laboratory’s mission.
References
Glatter, O., and O. Kratky. 1982. Small Angle X-Ray Scattering. London: Academic Press. doi: 10.1002/actp.1985.010360520.
Ilavsky, J., et al. 2013. "Ultra-Small-Angle X-ray Scattering Instrument at the Advanced Photon Source: History, Recent Development, and Current Status." Metallurgical and Materials Transactions A: Physical Metallurgy and Materials Science 44A (1):68—76. doi: 10.1007/s11661-012-1431-y.
Ilavsky, J., and P. R. Jemian. 2009. "Irena: Tool Suite for Modeling and Analysis of Small-Angle Scattering." Journal of Applied Crystallography 42: 347—353. doi: 10.1107/S0021889809002222.
Tappan, B. C., et al. 2010. "Nanoporous Metal Foams." Angewandte Chemie-International Edition 49 (27):4544—4565. doi: 10.1002/anie.200902994.
Publications and Presentations
Bagge-Hansen, M., P. G. Campbell, J. D. Colvin, T. E. Felter, and S. O. Kucheyev. 2015. Porous Materials Via Freeze-Casting Of Metal Salt Solutions. US Patent Application 14/741,334, filed June 16, 2015.
Qi, Z., et al. 2016. "Nanoporous Ag Powder via Freeze Drying: From Ice to Nanomaterials." Noble Metal Nanoparticles, Gordon Research Conference, South Hadley, MA, June 19—24, 2016. LLNL-POST-695359.