Patrick Campbell (13-ERD-056)
Abstract
We investigated benign lithium salts that can replace problematic lithium halide salts in the tritium recovery process of future fusion power plants, thereby avoiding corrosion and volatility problems. A new contactor was developed that eliminates the need for high-temperature, high-speed centrifugal contactors. This alternative approach and efficient tritium recovery process from lithium will have a major impact on the design, operation, and cost of tritium separation processes envisioned for virtually all planned fusion reactors. Records of invention and provisional patents have resulted from our research, thereby providing the Laboratory with a unique position in the event that the promise of the new process for tritium recovery is realized.
Background and Research Objectives
Laser inertial fusion energy can achieve a self-sustained fuel cycle by breeding tritium in a lithium blanket. The leading candidate for efficient tritium extraction is a lithium halide chemistry system that uses centrifugal contactors to extract tritium into the salt phase and then recovers it via electrolysis. The objective of this work was to develop a safe and reliable process for tritium recovery that replaces the volatile and corrosive halides with more benign chemical compounds and eliminates the need for rotating machinery. Key goals included demonstration of extraction feasibility; development of a design methodology for the electrolyzer unit, including development of computational fluid dynamics models; and advancing the development of a reliable non-centrifugal contactor to eliminate a key source of vibration and operational risk.
Scientific Approach and Accomplishments
In FY13 we (1) identified two halide-free salts, lithium hydroxide and lithium carbonate, and performed initial insolubility tests between metal and salt; (2) completed the conceptual design for the non-centrifugal contactor, including a distributor plate, an ultrasonic emulsifier to maximize interfacial area between phases, and a proposed hydrocyclone separator; (3) selected candidate surrogate fluids (hexane/water and water/HFE7500) that closely mimic the lithium/lithium-salt systems; (4) developed a non-dimensional analysis for the hydrocyclone separator that enables scaling between the lithium/lithium-salt and surrogate fluid systems; (5) constructed a preliminary plastic model for flow visualization to compare to computational fluid dynamics models; (6) created a single-phase model that shows maximum centripetal acceleration for a cylindrical-shape separator; and (7) completed a preliminary design for the electrolyzer.
In FY14, departure of key team members led to a reshaping of the team and a rescoping of the project. The team moved forward with new LLNL co-investigators and external collaborators from Argonne, Los Alamos, and Savannah River national laboratories. The rescoped project focused on completing theoretical models and associated analysis, as well as the initial demonstration of the alternative chemistry that would allow the replacement of the hazardous halides from the traditional process with benign lithium salts. Initially, a hydrocyclone concept was conceived as a potential two-in-one contactor and separator device for tritium removal from fusion-reactor lithium-coolant/tritium-breeder process streams. ANSYS Inc.’s computational fluid dynamics code CFX was used to verify and analyze hydrocyclone parameters and geometries provided by collaborators at Colorado State University in Fort Collins.1 Additional research was performed to locate material properties for the molten salt fluids under consideration for this analysis, specifically anhydrous lithium hydroxide and lithium carbonate. Little data exists for specific fluid mechanical properties for anhydrous lithium hydroxide, and initially viscosity data was varied over a collection of simulations to determine sensitivity of the viscosity value. Buoyancy and centrifugal forces were found to dominate the fluid species separation. As a result, simulated anhydrous lithium hydroxide viscosity variations between 0.08 and 2.0 cP did not have any significant effect on hydrocyclone effectiveness.
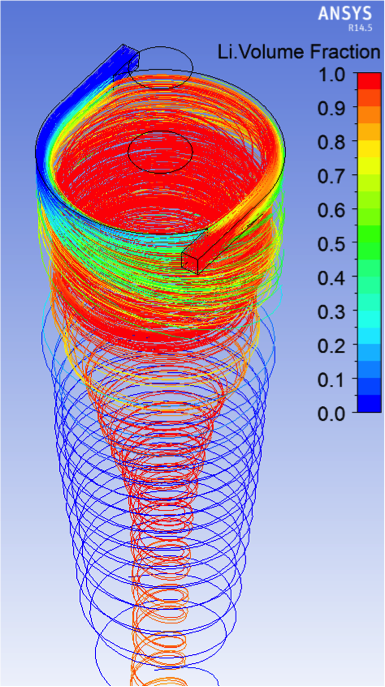
With two inlets—one molten salt and the other tritium-contaminated lithium from the fusion blanket—it was evident that ensuring the two fluids remain in contact for a meaningful length of time may be difficult. Analysis of simulated flow stream data (see Figure 1) over varying stream time lengths can be used to generate data trends for contact times, once specific fluid property data that must be gathered experimentally (e.g., interfacial tension) can be introduced to the modeling effort.
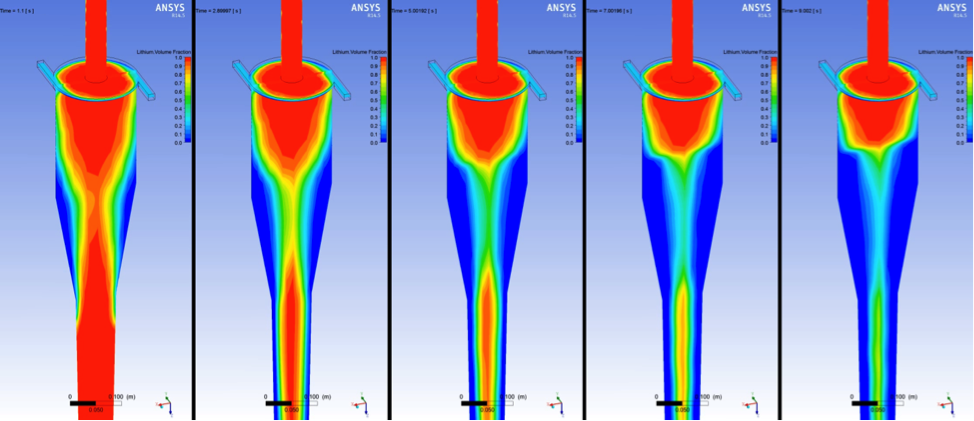
Without specific data, especially tritium transport within and between the fluid species, we determined that timescales required to achieve necessary transfer efficiencies may require a separate mixing-focused process prior to a hydrocyclone separation stage. A collaborator from Argonne National Laboratory provided us with modeling and simulation advice regarding several computational fluid dynamics setup and process parameters specific to contactor–separator operation. For example, specifying fluid ratios between the continuous phase and dispersed phase of 3:1 are typical for efficient contactor–separator systems. We used this ratio for fluid inlet volume fraction definitions for future hydrocyclone separator-only analyses. Figure 2 shows lithium–lithium-carbonate simulation time steps of a model that mimics the hydrocyclone geometries reported by Colorado State University.
Flow continues to develop toward a highly separated condition, although additional analysis will be required to fully determine single-stage efficiencies. Multiple hydrocyclone stages may be needed to ensure a final separation specification is satisfied. Sensitivities to fluid extraction rates between the light fluid overflow output and the heavy fluid underflow output became apparent during simulation set up and results analysis. Once the volume fractions of the two fluids approach their steady-state conditions, the prevention of excessive growth or collapse of the light fluid "bulb" collected at the overflow outlet (far right image of Figure 2) may present a challenge related to flow control schemes. Ultimately, the significant density difference between lithium (~460 kg/m3) and the molten salt (1,500–1,811 kg/m3) quickly develops into separated flows. Specific fluid mechanical properties and hydrogen isotope diffusivity and solubility at elevated temperatures, including two-species parameters such as interfacial tension and partition coefficients, are needed to fully analyze and drive the design of a complete contactor–separator system.
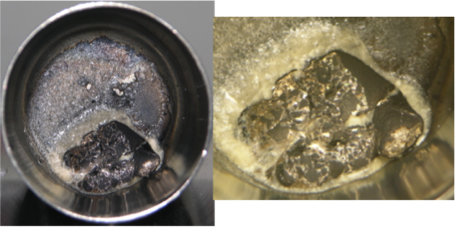
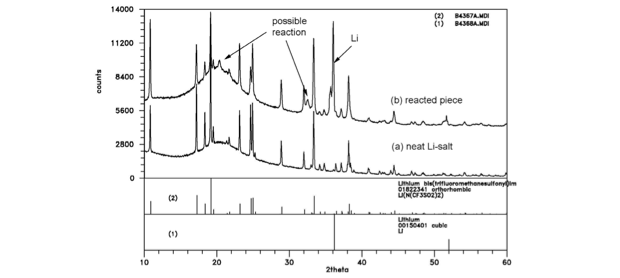
In FY15, we determined that anhydrous lithium hydroxide and lithium carbonate suffer from unfavorable reactions with the lithium blanket and focused our investigation on lithium bis(trifluoromethane)sulfonamide, which is a material widely used as an electrolyte in lithium-ion batteries and has remarkable temperature and chemical stability. We performed melt tests and chemical compatibility studies at temperatures up to 300°C, with favorable results (see Figures 3 and 4). Concurrently, our collaborators at Savannah River National Laboratory investigated rubidium chloride, an alkali metal halide that is promising as a tritium extraction salt because of a lower corrosion potential than other alkali halides, as well as having fewer activation products upon irradiation.
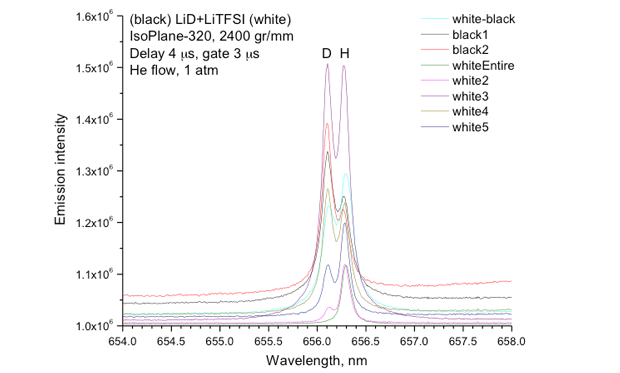
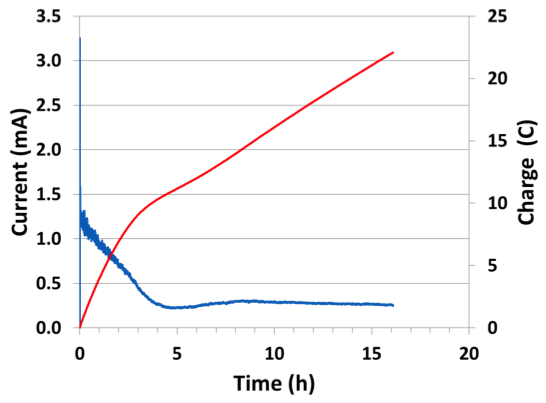
Previous determination of the tritium extraction efficiency of molten salts relied on radiation counting techniques. Because of the hazards of working with radioactive tritium, we used lithium hydride and lithium deuteride as surrogates. It can be quite challenging to perform quantitative analysis of hydrogen because its low mass and high abundance in the environment can lead to unreliable results using standard techniques. We collaborated with scientists at Applied Spectra in Fremont, California to utilize laser-induced breakdown spectroscopy to measure the deuterium content of samples of lithium bis(trifluoromethane)sulfonamide/lithium deuteride. We were able to easily separate the deuterium (sample) from hydrogen (background environment) using the laser-induced breakdown spectroscopy technique (Figure 5). Savannah River National Laboratory has implemented a gas chromatography setup inside a glove box to minimize stray background signals, and preliminary results are promising. Savannah River also demonstrated electrolysis of lithium hydride from molten rubidium-chloride–lithium-chloride with up to 92% conversion efficiency (see Figures 6 and 7).
Figure 7. Plot of current and charge versus time for 0.01 mol% lithium hydride (0.00183 g) in rubidium–chloride–lithium-chloride (22 C charge passed = 92% efficiency) for the high-temperature experiment shown in Figure 6.
Impact on Mission
The development of this next-generation tritium recovery process for a laser inertial fusion energy market-entry plant is key for the optimization of the fuel cycle and will ultimately allow for an attractive and self-sustained solution for closing the fusion fuel cycle. We have developed a new enabling technology for tritium recovery from fusion blankets, which will provide energy security benefits beyond laser inertial fusion energy to both the DOE and NNSA.
Conclusion
We have developed a new contactor based on a hydrocyclone design that eliminates the need for high-temperature, high-speed centrifugal contactors. We have investigated new lithium salts such as lithium bis(trifluoromethane)sulfonamide, which can replace problematic lithium halide salts in the tritium extraction process. We developed analytical systems to measure the extraction efficiency of the new molten salt systems that do not rely on counting radioactive tritium, and demonstrated proof of principle using model lithium bis(trifluoromethane)sulfonamideI and lithium deuteride mixtures. This successful research project has resulted in records of invention and provisional patents. The next step would be to fully characterize the extraction performance of the new salt systems.
References
- Bandhauer, T., and J. Adler, Alternative methods for trituim recovery from the LIFE lithium blanket: FY13 final report. Colorado State University, Fort Collins, CO. (2013).
Publications and Presentations
- Reyes, S., “LIFE: A sustainable solution for developing safe, clean fusion power.” Health Phys. 104, 641 (2013). LLNL-TR-576952.
- Reyes, S., et al., “LIFE tritium processing: A sustainable solution for closing the fusion fuel cycle.” Fusion Sci. Tech. 64, 187 (2013). LLNL-TR-576952.