Katelyn Mason | 18-FS-006
Overview
Confirming exposure to chemical warfare agents (CWA) is necessary for forensic efforts aimed at reliably determining their use, detecting production, and informing diagnostic procedures that guide medical countermeasures. Human CWA exposure is traditionally determined by identifying biomarkers such as metabolites, deoxyribonucleic acid (DNA) adducts, and protein adducts found in biological liquids (urine, blood, and plasma). Detection of these biomarkers is limited by time (hours to months) due to metabolic turnover and detoxification mechanisms. Additional challenges include sample preservation caused by chemical degradation. The identification of long-term (years to lifetime) biomarkers would increase the time available for exposure confirmation and consequently the number of events amenable to investigations. The strategy implemented in this study establishes a framework for understanding if chronic low-level CWA exposure elicits an immune response in humans. In theory, there are lifetime-detectable immune changes that can reveal CWA exposure. Protein adducts created by reactions with CWAs during exposure can serve as antigens targeted by immune response mechanisms, and immunogenic antibodies specific to CWA-adducted proteins might be exploited as permanent biomarkers. In forensic practice, surfaces functionalized by an antigenic CWA-protein adduct can be used to detect CWA exposure by binding to an antibody. We performed a multistage study focusing on the blister agents sulfur mustard and nitrogen mustard. The first stage identified new protein adducts resulting from exposing purified plasma proteins and human plasma with different mustards. Adducts were detected using traditional proteomic sample preparation, nanoflow liquid chromatography-based mass spectrometry, and software. The second stage was the production of an antibody specific to a CWA-adducted peptide using an alternative biological system (rabbit) as a surrogate to humans. The third stage was testing the ability of the antibody to bind the antigen (CWA-adducted peptide). Results from this study established the feasibility of exploiting antibodies as long-timescale biomarkers of CWA exposure and indicated a unique strategy for simple exposure testing.
Background and Research Objectives
The lifetime of biomarkers used in conventional techniques is often shorter than the time span between exposure and sample collection (Smith et al. 2008). Human CWA exposure is currently determined by identifying metabolites, DNA adducts, and protein adducts found in internal biological liquids (e.g. urine, blood, and plasma (Black 2010, Pantazides et al. 2015, Nie et al. 2011). Biomarkers found in biological fluids are subject to metabolic turnover, detoxification mechanisms, and chemical degradation (Black 2010, Noort et al. 1999). The persistence of specific biomarkers in biological samples possesses variable time frames, and in all cases, the concentration of target compounds decreases over time, often resulting in small concentrations (in the parts-per-billion range) that are difficult to detect. The need to establish new long-lived biomarkers is essential to properly confirming CWA use in realistic time frames. By focusing only on established biomarkers, standard practices leave a large gap that can be filled by research for new CWA-related biomarkers and the development of new methodologies aimed at detection over longer time frames.
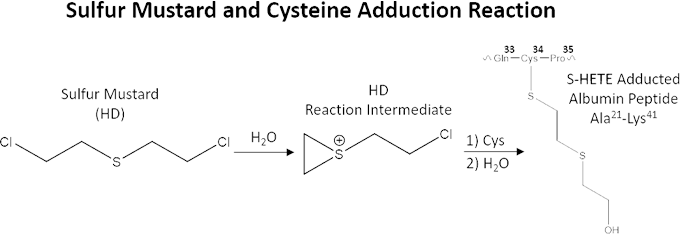
Antibodies created by immune response mechanisms circulate in an individual’s blood system to detect the presence of targeted antigens for subsequent destruction (Boes 2000). These immunogenic antibodies have longer lifetimes (years to lifetime) than traditional CWA-exposure biomarkers used by conventional methods of detection because, unlike other proteins present in plasma, their production is maintained (Slifka et al. 1995). If CWA-specific antibodies exist, they can provide novel biomarkers that can be used to detect CWA exposure over long time periods. CWAs are known to modify proteins upon contact by creating an adduct connected to specific amino acid side chains (Noort et al. 1999, 2002, 2004). The figure below shows an example of the reaction between sulfur mustard and the amino acid cysteine (amino acid number 34 in human serum albumin). Modified and adducted biological macromolecules have been shown to result in the production of adduct-specific antibodies in numerous studies probing the outcomes of their influence on the immune system (Luster and Rosenthal 1993, Willis et al. 2002, Shaw et al. 2000). Exposure to harmful chemicals can have a profound effect on the immune system although it is still not completely understood (Colosio et al. 2005). Previous research indicates there is an increase in antibody production in humans exposed to organophosphate pesticides, which are members of the same class of organophosphorus chemicals as nerve agents, such as VX and sarin (Corsini et al. 2013).
Sulfur mustard (HD) reaction with albumin protein cysteine (cys) 34 and resulting sulfur-hydroxyethylthioethyl (S-HETE) adduct. Amino acids are abbreviated to a three-letter code (Gln = glutamine; Cys = cysteine; Pro = proline; Ala: alanine; Lys = lysine) and a superscript number that shows the protein’s position in the amino acid sequence.
In the event of a release of a CWA and acute exposure, biomedical testing for biomarkers is imperative for confirmation and for gauging the risk posed to global security and human health. In cases of chronic low-level exposure to CWAs, potential antigens (adducted proteins) constantly circulate in the blood system and can possibly elicit the production of corresponding antibodies; therefore, antibodies have the potential to serve as a long-term marker that indicates low-level chronic exposures to CWAs. The purpose of this study, then, was to probe potential immune responses to chronic low-level CWA exposure. To do this, we sought to establish a new suite of CWA-specific protein adducts, obtain an adduct-specific antibody, and test its ability to bind the adducted peptide antigen. The determination of whether the blood plasma proteins that have been modified by CWAs can be probed by antibody binding provides the essential ground work to further explore if their presence in blood can be used to confirm CWA exposure on a long timescale.
This feasibility study was successful in identifying a new suite of potential biomarkers resulting from mustard CWA exposure. A specific peptide-adduct antibody was also developed to serve as a surrogate to endogenous antibodies created by sulfur mustard CWA exposure. This antibody can additionally be used to purify targeted adducted-peptides from suspected CWA-exposed samples for a more sensitive detection assay. Ultimately, this study established the ground work for the testing and detection of CWA-specific antibodies that persist for long periods of time, produced from acute and low-level chronic exposure to CWAs.
Impact on Mission
This study addresses Lawrence Livermore National Laboratory’s research challenges in the fields of forensic science and chemical and biological countermeasures. Specifically, the aims of this study directly address understanding exposure signatures of current and emerging chemical threats. Results of this research enable improvements for determining chemical warfare agent exposure levels, protein signatures, and their timelines. In addition to the large set of chemical agents targeted in this study, this strategy can be applied to understanding exposure to other CWAs and allows for the development of a field-applicable analytical surveillance technique that does not require a mass spectrometer. In the instance of additional funding and research, the direction established in this study could ultimately result in the invention of a novel methodology for CWA exposure forensics that would set Lawrence Livermore’s forensic science work apart from other forensic laboratories.
Conclusion
The value of identifying long-term biomarkers and developing a technique for their detection cannot be overstated. The ability to detect CWA exposure over long periods of time could be a game changer for U.S. military- and humanitarian-led medical event attribution efforts. Through this study, we not only initiated and demonstrated a novel approach to CWA detection, but that approach, which uses antibodies, has a superior limit of detection due to the sensitivity of fluorescence measurements.
Looking forward, a field-ready strategy could also be developed from this study that eliminates the use of mass spectrometers that are difficult to bring into the field. With further funding, other CWAs (chlorine gas, nerve agents, and others) could be explored using this approach. Indeed, the chemicals applicable to this project are not limited to CWAs. Further work might also include the identification and detection of long-term biomarkers associated with chronic exposure to reaction precursors. This would provide a unique opportunity for establishing links between individuals and laboratories where CWAs are illegally produced. This additional layer of valuable information regarding reaction precursor and/or byproduct exposure is otherwise lost when only probing short-lived biomarkers from the CWA itself, as they often degrade prior to obtaining biological samples.
Applications for this technology include but are not limited to the numerous circumstances where confirmation of CWA use or exposure is necessary for deciding medical treatment, finding individuals who are participating in CWA synthesis, and assisting in cases where biological samples cannot be collected in the short time frames typically needed for medical event attribution.
References
Black, R. M. 2010. "History and Perspectives of Bioanalytical Methods for Chemical Warfare Agent Detection." Journal of Chromatography B 878 (17–18):1207–1215.
Boes, M. 2000. "Role of Natural and Immune IgM Antibodies in Immune Responses." Molecular Immunology 37 (18):1141–1149.
Colosio, C., et al. 2005. "Low Level Exposure to Chemicals and Immune System." Toxicology and Applied Pharmacology 207 (2):320–328.
Corsini, E., et al. 2013. "Pesticide Induced Immunotoxicity in Humans: A Comprehensive Review of the Existing Evidence." Toxicology 307:123–135.
Luster, M. I. and G. J. Rosenthal. 1993. "Chemical Agents and the Immune Response." Environmental Health Perspectives 100:219.
Nie, Z., et al. 2011. "Improvements in Monitoring the N-Terminal Valine Adduct in Human Globin After Exposure to Sulfur Mustard and Synthesis of Reference Chemicals." Talanta 85 (2):1154–1159.
Noort, D., et al. 1999. "Alkylation of Human Serum Albumin by Sulfur Mustard In Vitro and In Vivo: Mass Spectrometric Analysis of a Cysteine Adduct as a Sensitive Biomarker of Exposure." Chemical Research in Toxicology 12 (8):715–721.
——— . 2002. "Covalent Binding Of Nitrogen Mustards to the Cysteine-34 Residue in Human Serum Albumin." Archives of Toxicology 76 (2):83–88.
——— . 2004. "Retrospective Detection of Exposure to Sulfur Mustard: Improvements on an Assay for Liquid Chromatography-Tandem Mass Spectrometry Analysis of Albumin/Sulfur Mustard Adducts." Journal of Analytical Toxicology 28 (5):333–338.
Pantazides, B. G., et al. 2015. "Simplified Method for Quantifying Sulfur Mustard Adducts to Blood Proteins by Ultrahigh Pressure Liquid Chromatography–Isotope Dilution Tandem Mass Spectrometry." Chemical Research in Toxicology 28 (2):256–261.
Shaw, P. X., et al. 2000. "Natural Antibodies with the T15 Idiotype May Act in Atherosclerosis, Apoptotic Clearance, and Protective Immunity." The Journal of Clinical Investigation 105 (12):1731–1740.
Slifka, M. K., et al. 1995. "Bone Marrow Is a Major Site of Long-Term Antibody Production After Acute Viral Infection." Journal of Virology 69 (3):1895–1902.
Smith, J. R., et al. 2008. "Analysis for Plasma Protein Biomarkers Following an Accidental Human Exposure to Sulfur Mustard." Journal of Analytical Toxicology 32 (1):17–24.
Willis, M. S., et al. 2002. "Adduction of Soluble Proteins with Malondialdehyde-Acetaldehyde (MAA) Induces Antibody Production and enhances T-Cell Proliferation." Alcoholism: Clinical and Experimental Research 26 (1):94–106.