Brandon C. Wood (15-ERD-022)
Abstract
All-solid-state batteries promise safer, more reliable performance compared to existing ion-battery technologies based on liquid and polymer-gel electrolytes. Using a solid electrolyte replacement can also enable the use of higher-voltage cathodes and metal anodes to dramatically enhance battery capacity. Unfortunately, no solid electrolyte has yet achieved the combination of high conductivity, electrochemical stability, and low interfacial resistance necessary for widescale commercial deployment. A key limitation is the lack of fundamental understanding of the underlying characteristics and materials properties that motivate fast ionic conductivity in solids. This project addressed this gap by integrating theoretical simulation techniques across multiple scales and by combining theory with experiments to extract the structure-property relationships that govern ionic conductivity. Our efforts focused on three objectives. First, a “horizontal” approach was implemented that uses high-throughput computational “experiments” to test diffusion under various conditions. This led to a new understanding of how different chemical and structural factors affect ionic conductivity in borohydride-based solid electrolytes. Second, we performed an in-depth “vertical” analysis of the mechanisms and motivations for ionic conductivity in several diffusive materials, including borohydride- and halide-based conductors. We discovered several new mechanisms that have never been reported and used these to develop novel descriptors for predicting ionic conductivity. Third, with guidance from experiments, we worked to integrate ab initio and mesoscale modeling techniques to predict conductivity under more realistic device operational conditions. These simulations focused on understanding the effects of interfaces, structural disorder, and grain boundaries; they resulted in new modeling capabilities and methods that can be more widely applied to meet Laboratory objectives. In addition to the scientific contributions, this report describes several new partnerships with national laboratories, universities, and industry stakeholders.
Background and Research Objectives
Solid electrolytes with high ionic conductivity are crucial for developing batteries and membranes with dramatically improved mechanical, thermal, and electrochemical stability (Knauth 2009). All-solid-state batteries could also achieve higher voltages and capacities more safely than volatile liquid electrolytes. Viable solid electrolytes must be “superionic,” achieving liquid-like cation conductivity while retaining a stable solid framework. Unfortunately, poor understanding of the physiochemical factors that regulate ionic conductivity has inhibited rational design strategies for solid electrolytes. As a result, discovery of new solid electrolytes has been largely Edisonian in nature, and overcoming conductivity and stability limitations in existing materials has proven difficult.
The goal of this project was to establish an integrated approach for understanding and predicting high ionic conductivity in materials at multiple scales, thereby enabling improved descriptors for screening and optimizing solid electrolyte candidates. For this project, the researchers pursued and met three specific objectives:
- develop a “horizontal” data-driven approach to probing structure-property correlations,
- develop a “vertical” approach for detailed probing of diffusion mechanisms, and
- predict and improve macroscopic conductivity under operation.
The first objective focused on high-throughput computational “experiments” to understand how ionic conductivity is affected by structural and chemical factors. The second objective explored key mechanisms to propose new descriptors for tuning ionic conductivity. The third objective involved the development of new multiscale methods for probing ionic conductivity within more realistic materials and microstructures. New external collaborations introduced later in the project expanded the original scope to encompass novel solid-electrolyte materials and deeper investigations into interfaces.
Scientific Approach and Accomplishments
This project integrated a variety of theoretical techniques spanning a range of length and time scales, from density functional theory calculations and ab initio molecular dynamics (AIMD) for Objectives 1 and 2, to phase-field simulations and kinetic-transport modeling for Objective 3. Several spectroscopic- and electrochemical-characterization techniques were also employed. The key accomplishments are organized (below) according to the three project objectives.
Objective 1: The “Horizontal” Data-Driven Approach for Structure-Property Correlations
Automated platform for "high-throughput" AIMD. At the heart of our project was the acquisition and analysis of a comprehensive set of AIMD simulations to readily survey a large number of materials and material-specific perturbations. We developed a Python-based modular workflow environment for reading in-structure information, generating the relevant input files and parameters, and automating the job submission and data analysis. These codes were built around the Quantum Espresso AIMD code (Giannozzi et al. 2009) and designed for integration into the AiiDA high-throughput platform developed at the École Polytechnique Fédérale de Lausanne (EPFL; Pizzi et al. 2016). As a future endeavor, we have partnered with machine-learning and data-science efforts at Sandia National Laboratories and Stanford University to highlight trends and correlations in our aggregated data.
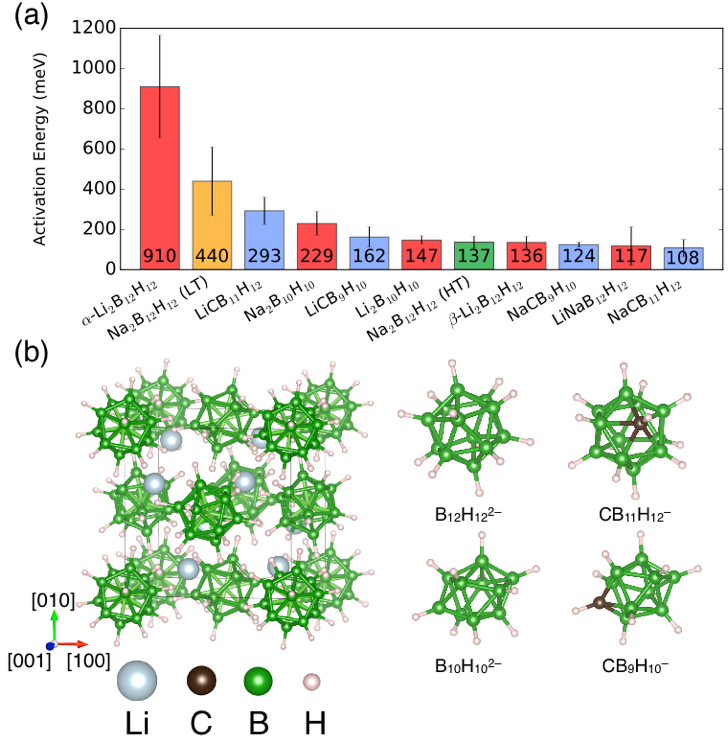
Broad survey of conductivity trends in borohydride ionic conductors. We partnered with NIST and the Sandia National Laboratories on the development of closo-borate solid electrolytes, a new class of exceptional ionic conductors (Mohtadi and Orimo 2016; Udovic et al. 2014; Tang et al. 2015). These materials contain B12H122-, B10H102-, and their relatives as large stabilizing anions. We used our automated AIMD framework to collect data from a large set of these materials, varying the cation and molecular anion species, as well as the crystal structure (Figure 1). Our results showed no clear correlation of conductivity to crystal structure, but identified a strong sensitivity of the results to the material’s cation density and volume. Point defects such as lithium vacancies and carbon substitution for boron also favorably influenced the ionic mobility. Volume expansion (e.g., via alloying) can favorably assist diffusivity, but only to a limit whose value depends on the fundamental nature of the cation-anion interaction (Varley et al. 2016).
Objective 2: The “Vertical” Approach for Detailed Probing of Diffusion Mechanisms
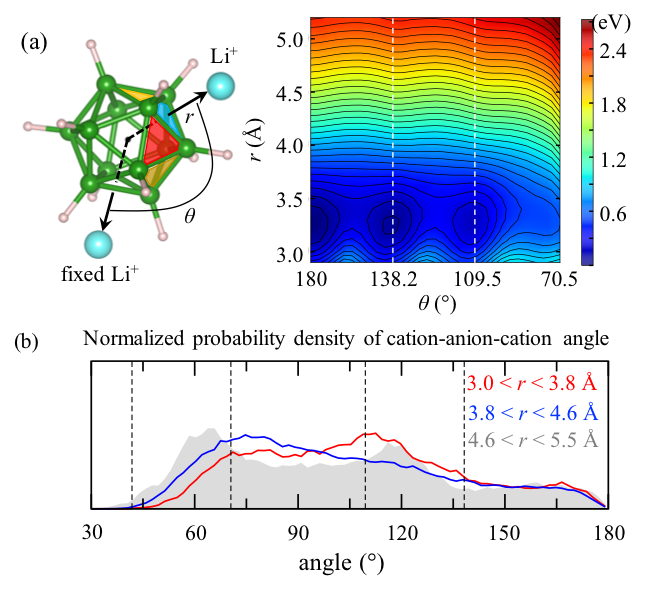
Novel diffusion mechanisms and descriptors in closo-borate ionic conductors. As part of our detailed probe of mechanisms, we performed additional in-depth studies of the closo-borate salts Li2B12H12 and Na2B12H12. The anions in these materials tend to have high rotational mobility that appears to correlate with cation diffusion (Verdal et al. 2014). We developed a new analysis toolkit that examines anion orientational preferences, rotation rates, and librational frequencies within the lattice. We found that superionic behavior in Li2B12H12 and Na2B12H12 results from a combination of key structural, chemical, and dynamic factors that introduce intrinsic frustration and disorder. We introduced a new statistical metric based on cation probability density as a descriptor and showed that the density of accessible interstitial sites can correlate with observed cation mobility. We also found that the B12H122- anions show a strong orientational preference when binding closely coordinated Li+ (Figure 2). This suggests that nearby cations will tend to reflect the geometry of the anion and dock at preferred sites. However, this anion geometry is incompatible with the crystallographic interstitial-site preference, which leads to a competition that can facilitate fast cation hopping. The fast anion rotations dynamically alter the energy landscape, creating an additional local driving force that helps cations escape local trapping. The findings were used to suggest several new descriptors for ionic conductivity that had not yet been reported (Kweon et al. 2017).
Complex dynamics in carba-closo-borate ionic conductors. Our analysis was also applied to study anion dynamics in the carba-closo-borates LiCB11H12 and NaCB11H12, which feature even faster ionic conductivity than their Li2B12H12 and Na2B12H12 cousins (Tang et al. 2016). Partnering with the National Institute of Standards and Technology (NIST), we combined our AIMD analysis with quasielastic neutron scattering (QENS) (Copley and Cook 2003), adding a new capability to simulate the QENS elastic incoherent structure factors from AIMD for one-to-one comparisons between the two. We found that the symmetry-breaking carbon atom on the CB11H12- icosahedron perturbs the energy landscape along the instantaneous orientation of the anion dipole, which further strengthens the coupling between the fluctuations in the cation probability density and the anion reorientational motion. This synergy between the anion reorientational dynamics and the carbon-modified anion dipole was found to be primarily responsible for the higher ionic conductivity observed in salts based on CB11H12- compared with B12H122-. This suggests that modifying the anion could be a promising strategy for improving ionic conductivity (Dmitrievska et al. 2017).
Novel diffusion mechanisms in halide ionic conductors. We performed AIMD simulations on Li3InBr6 (Tomita et al. 1998) to explore the interplay between chemistry, structure, and ionic conductivity in halide solid electrolytes, which are promising because they tend to blend high ionic conductivity with high stability. We partnered with San Francisco State University to introduce a novel analysis approach based on maximally localized Wannier functions (Marzari et al. 2012), which assigns “positions” to key features in the electronic charge density to track how chemical bonding and polarization evolve as ions migrate. We found evidence of rapid fluctuations between bonding states with distinctly different degrees of directional covalent character, implying that the cation-anion interactions are not simple ionic bonds. The covalent-bond character leads to local directional preferences that are incompatible with the lattice symmetry, which causes the bonding states to fluctuate in a manner that drives the diffusion of Li+ (Adelstein and Wood 2016). Follow-up work on Li3InBr6-xClx alloys showed that combining different halide ions can favorably alter the cation-anion chemistries, opening up additional avenues for improving halide conductors (Zevgolis et al. 2017).
Objective 3: Predicting and Improving Macroscopic Conductivity Under Operation
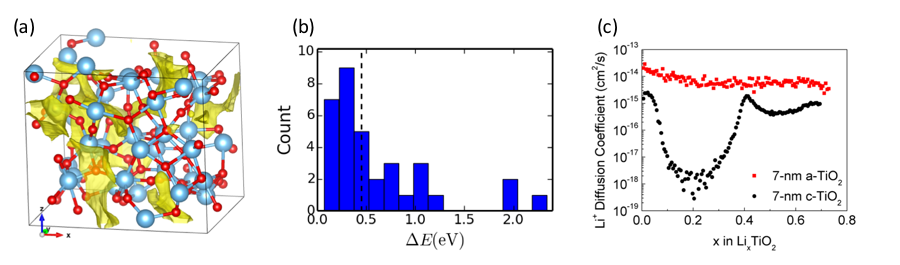
Understanding ionic conductivity in disordered and amorphous systems. To better understand how disorder affects ionic mobility, we ran a combined experiment-theory study of Li+ diffusion in TiO2, using a controlled synthesis platform based on atomic layer deposition to tune the amorphous character of the material. The results showed that the amorphization can lead to much faster Li+ diffusion. Using AIMD, we generated validated models of amorphous TiO2, which were used to investigate the energy landscape for cation diffusion (Figure 3). Partnering with EPFL and the University of Notre Dame to locate sites and pathways in the amorphous structure, we found that low barriers are present between some Li+ environments due to the intrinsic structural disorder of amorphous TiO2. In thin films, these sites can form low-barrier pathways that can lead to significantly improved rate performance in thin films. This suggests that amorphous materials may be a viable alternative to crystalline layered materials for high-rate-performance energy storage (Ye et al. 2017).
Effects of defects on ionic transport. The effects of defects and vacancies on ionic transport were studied using defective graphene in an anode configuration, with systematic hydrogen dosing and thermal treatment, both used to introduce controlled defect concentrations. It was shown that hydrogen can attack high-energy sites such as grain boundaries, opening up additional transport channels that dramatically accelerate Li+ diffusion. A new kinetic model was devised to explain this process and derive optimized exposure conditions for defect creation based on model energetics. Although every material is unique in how vacancies and defects affect ion transport, the theory-experiment integration and kinetic-modeling activities developed within this task are broadly applicable (Ye et al. 2015).
Modeling polycrystalline systems. Real solid electrolytes can have complex microstructures that differ significantly from ideal single crystals, particularly near electrode interfaces. We developed a new statistical method for accounting for the role of interfacial structural defects in materials with interfacial strain associated with buildup of cation concentration, which can occur near the interface with an electrode due to electric fields. Our method considers the role of defects in alleviating strain within the context of microelasticity theory. We tested the method on the electrode material LiFePO4 (Padhi et al. 1997), for which microstructural information was available. We were able to resolve longstanding discrepancies regarding the orientations of phase boundaries in this system, which we attributed to configurational entropy arising from defects at interfaces (Heo et al. 2016).
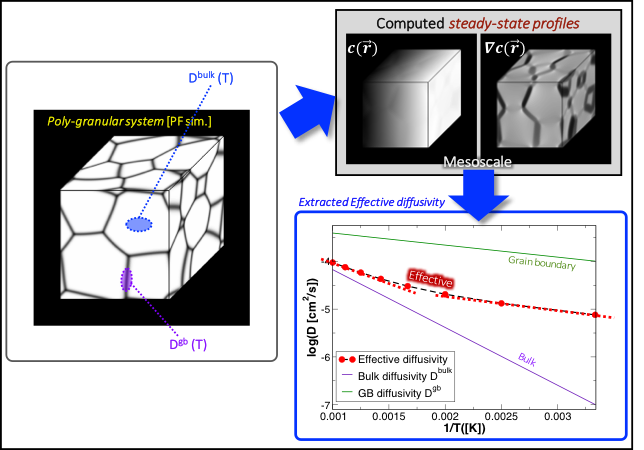
We also established a new modeling framework for computing ionic conductivity in the presence of multiple grains or phases (Figure 4). We first generated a relevant microstructure using a phase-field simulation (Krill and Chen 2002; Heo and Chen 2014), then assigned separate ion diffusivities to bulk and grain/phase boundary portions from atomistic calculations. The steady-state concentration profile within the microstructure under an applied concentration gradient is then efficiently computed by the Fourier-spectral iterative perturbation numerical method (Zhu et al. 2001), from which the ionic diffusivity is extracted. The framework was used to compute the effective Li+ diffusivity through polygranular Li7La3Zr2O12 solid electrolytes (Knauth 2009), demonstrating that non-Arrhenius behavior can be traced to the presence of multiple grains. To inform the grain-boundary conductivity, we initiated a collaboration with the University of Michigan and San Francisco State University. Our collaborators are now continuing these activities, with the goal of joint research into the microstructure effects in solid-state batteries. We also applied our framework to Li3xLa2/3-2xTiO3 (Knauth 2009) to explore the effects of mesoscale boundaries between differently-aligned crystallite domains.
Figure 4. Schematic of our modeling framework for computing effective ion diffusion in complex microstructures. Diffusion coefficients derived from AIMD for bulk and grain-boundary regions are incorporated into a microstructural model, from which a steady-state concentration profile (top right) is computed within the phase-field simulation in response to an applied concentration gradient. The response function is used to derive the effective diffusivity (bottom right).
Impact on Mission
This project supported the Laboratory mission focus areas in energy security and scientific discovery, while applying core competencies in high-performance computing, energy and climate, and advanced materials. The project has already secured new funding avenues with industry and universities, ensuring a leadership role for the Laboratory in the simulation of solid-state electrolytes. It also incubated the development of several new general multiscale computational capabilities for predicting and understanding ionic conductivity, including a high-throughput task management engine for AIMD transport simulations, new descriptors for optimizing ionic conductivity, new analysis tools, new methods for interpreting QENS, and techniques for understanding defects and interfaces in mass transport processes. These capabilities may be broadly applied to a wide variety of mission activities.
Conclusion
The capabilities and understanding developed during this project are the basis of new and emerging activities, including three projects that have already been awarded. First, we began pursuing a Strategic Partnership Program (SPP) arrangement with an industrial partner. Second, the mass-transport methods were used as a focus capability for the Laboratory’s involvement in the new DOE-EERE Hydrogen Storage Materials—Advanced Research Consortium (HyMARC), through which our microstructure-dependent mass-transport models will be applied. Third, a National Science Foundation grant was issued to Professor Adelstein of San Francisco State University to work collaboratively with the Laboratory on future development of methods for ionic conductivity. We have also established new collaborations with Carnegie Mellon University and Stanford University on electrode-electrolyte interfaces; EPFL, Bosch, and University of Notre Dame on high-throughput computation for mechanistic discovery; NIST and Sandia National Laboratories for closo-borate solid electrolyte development; University of Michigan and Oak Ridge National Laboratory for microstructural effects on transport; and Georgia Institute of Technology and the Army Research Laboratory on halide conductors.
References
Adelstein, Nicole, and B. C. Wood. 2016. “Role of Dynamically Frustrated Bond Disorder in a Li+ Superionic Solid Electrolyte.” Chemistry of Materials 28: 7218. LLNL-JRNL-684599.
Copley, J. R. D., and J. C. Cook. 2003. "The Disk Chopper Spectrometer at NIST: A New Instrument for Quasielastic Neutron Scattering Studies." Chemical Physics 292: 477.
Dmitrievska, M., et al. 2017.“Effects of Carbon Incorporation and Anion Dynamics on Ultrafast Cation Diffusion in Superionic LiCB11H12 and NaCB11H12.” Submitted for review. LLNL-JRNL-740042.
Giannozzi, P. et al. 2009. “QUANTUM ESPRESSO: A Modular and Open-Source Software Project for Quantum Simulations of Materials.” Journal of Physics: Condensed Matter 21: 395502.
Heo, T. W., and L.-Q. Chen. 2014. "Phase-Field Modeling of Displacive Phase Transformations in Elastically Anisotropic and Inhomogeneous Polycrystals." Acta Materialia 76: 68. LLNL-JRNL-651372.
Heo, T. W., et al. 2016. “Defects,Eentropy, and the Stabilization of Alternative Phase Boundaries in Battery Electrode Particles.” Advanced Energy Materials 6: 1501759.
Kweon, K. E., et al. Forthcoming. “Structural, Chemical, and Dynamical Frustration: Origins of Superionic Conductivity in Closo-Borate Solid Electrolytes.” Chemistry of Materials. LLNL-JRNL-734199.
Knauth, P. 2009. “Inorganic Solid Li Ion Conductors: An Overview.” Solid State Ionics 180: 911.
Krill III, C. E., and L-Q. Chen. 2002. "Computer Simulation of 3-D Grain Growth Using a Phase-Field Model." Acta Materialia 50: 3059.
Marzari, N., et al. 2012. "Maximally Localized Wannier Functions: Theory and Applications." Reviews of Modern Physics 84: 1419.
Mohtadi, R., and S. Orimo. 2016. "The Renaissance of Hydrides as Energy Materials." Nature Reviews Materials 2: 16091.
Padhi, A. K., et al. 1997. "Phospho-Olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries." Journal of the Electrochemical Society 144: 1188.
Pizzi, G., et al. 2016. “AiiDA: Automated Interactive Infrastructure and Database for Computational Science.” Computational Materials Science 111: 218.
Tang, W. S., et al. 2015. "Unparalleled Lithium and Sodium Superionic Conduction in Solid Electrolytes with Large Monovalent Cage-Like Anions." Energy & Environmental Science 8: 3637.
———. 2016. "Stabilizing Superionic-Conducting Structures via Mixed-Anion Solid Solutions of Monocarba-Closo-Borate Salts." ACS Energy Letters 1: 659.
Tomita, Y., et al. 1998. "New Lithium Ion Conductor Li3InBr6 Studied by 7Li NMR." Chemistry Letters 27: 223.
Udovic, T. J., et al. 2014. "Sodium Superionic Conduction in Na2B12H12." Chemical Communications 50: 3750.
Varley, J. B., et al. 2017.“Understanding Ionic Conductivity Trends in Polyborane Solid Electrolytes from ab initio Molecular Dynamics.” ACS Energy Letters 2: 250. LLNL-JRNL-711522.
Verdal, N., et al. 2014. "Anion Reorientations in the Superionic Conducting Phase of Na2B12H12." The Journal of Physical Chemistry C 118: 17483.
Ye, J., et al. 2015. “Universal Roles of Hydrogen in Electrochemical Performance of Graphene: High Rate Capacity and Atomistic Origins.” Scientific Reports 5: 16190. LNL-JRNL-732977.
———. 2017. “Pseudocapacitive Fast Charge/Discharge in Atomic Layer Deposition-Derived Amorphous Titania.” Submitted for review. LLNL-JRNL-732977.
Zevgolis, A., et al. 2017. “Alloying Effects on Superionic Conductivity in Lithium Indium Halides for All-Solid-State Batteries.” Submitted for review. LLNL-JRNL-740955.
Zhu, J., et al. 2001. "Computing the Effective Diffusivity Using a Spectral Method." Materials Science and Engineering: A 311: 135.
Publications and Presentations
Adelstein, N. 2014.“First-Principles Molecular Dynamics Transport in Li3InBr6: Tools for High-Throughput Screening.” Multiscale Materials Modeling Conference, Berkeley, CA, October 2014. LLNL-PRES-653 669.
———. 2015a. “Competition Between Covalent and Ionic Bonding Drive Li Conductivity in Li3InBr6: Electronic Frustration in Superionic Electrolytes.” LLNL Postdoc Poster Symposium, June 2015. LLNL-POST-672855.
———. 2015b. “Dependence of Li Conduction in Solid Electrolytes on the Local Electronic Structure and Bonding.” Materials Research Society Spring Meeting, San Francisco, CA, April 2015. LLNL-ABS-663441.
———. 2016. “Electronic Frustration-Driven Ionic Conductivity in a Superionic Solid Electrolyte: Simulating Dynamic Disordering of Polar Covalent Bonds.” American Chemical Society National Meeting, San Diego, CA, April 2016. LLNL-PRES-690937.
———. 2017. “Subcontract Report: Diffusion Mechanisms and Bond Dynamics in Solid Electrolyte Ion Conductors.” LLNL Subcontract Report. LLNL-SR-739549.
Adelstein, N., and B. C. Wood. 2016. “Role of Dynamically Frustrated Bond Disorder in a Li+ Superionic Solid Electrolyte.” Chemistry of Materials 28: 7218. LLNL-JRNL-684599.
Bercx, M. 2017.“First-Principles Investigations of Local Cation-Anion Interactions in Polyborane Solid Electrolytes.” LLNL Summer Student Poster Symposium, August 2017. LLNL-POST-735500.
Dmitrievska, M., et al. 2017. “Effects of Carbon Incorporation and Anion Dynamics on Ultrafast Cation Diffusion in Superionic LiCB11H12 and NaCB11H12.” Submitted for review. LLNL-JRNL-740042.
Heo, T. W. 2015. “Effects of Interfacial Coherency on Phase Boundary Orientations in Phase-Separating Electrode Particles for Li-Ion Batteries.” Materials Research Society Spring Meeting, San Francisco, CA, April 2015. LLNL-PRES-669188.
Heo, T. W., et al. 2016. “Defects, Entropy, and the Stabilization of Alternative Phase Boundaries in Battery Electrode Particles.” Advanced Energy Materials 6: 1501759. LLNL-JRNL-673242.
———. 2017. “Effects of Mesoscopic Microstructural Features on the Effective Ionic Diffusivity of Solid Electrolytes for Li Batteries.” Electrochemical Society Meeting, New Orleans, LA, May 2017. LLNL- PRES-731795.
Kweon, K. E. 2017. “Li and Na Superionic Conduction in Closo-Borane Solid Electrolytes.” LLNL Postdoc Poster Symposium, June 2017. LLNL-POST-733099.
Kweon, K. E., et al. 2017. “Structural, Chemical, and Dynamical Frustration: Origins of Superionic Conductivity in Closo-Borate Solid Electrolytes.” Chemistry of Materials 29: 9142. LLNL-JRNL-734199.
Mehta, P. “Unlocking Hidden Factors Controlling Conductivity in Superionic Solid State Electrolytes.” LLNL Summer Student Poster Symposium, August 2016. LLNL-POST-698944.
Shea, P. T. 2016. “Diffusion of Lithium in Titanium Oxide.” American Physical Society March Meeting, Baltimore, MD, March 2016. LLNL-PRES-685844.
———. 2017a. “Modeling Liquid-Like Ionic Conductivity in Closoborane Solid Electrolytes.” LLNL Postdoc Poster Symposium, June 2017. LLNL-POST-733085.
———. 2017b. “Investigating the Mechanism for High Ionic Conductivity in Polyborane Solid Electrolytes.” Materials Research Society Fall Meeting, Boston, MA, November 2017. LLNL-PRES-742407.
Varley, J. B. 2016a. “First-principles Investigations of Ionic Conduction in Li and Na Borohydrides.” American Physical Society March Meeting, Baltimore, MD, March 2016. LLNL-PRES-685851.
———. 2016b. “Mechanistic Insights into the Alkali Conduction Mechanisms of Closoborane Solid Electrolytes.” Materials Research Society Fall Meeting, Boston, MA, November 2016. LLNL-ABS-695419.
———, et al. 2017. “Understanding Ionic Conductivity Trends in Polyborane Solid Electrolytes from ab initio Molecular Dynamics.” ACS Energy Letters 2: 250. LLNL-JRNL-711522.
———. 2016a. “Ab initio Simulations of Charged Interface Effects in Graphene-Based Supercapacitors.” American Chemical Society Meeting, San Diego, CA, March 2016. LLNL-PRES-685853.
———. 2016b. “Interfacial Effects in the Charging/Discharging of Graphene Supercapacitors.” Telluride Workshop on Interfacial Chemistry and Charge Transfer for Energy Storage and Conversion, Telluride, CO, July 2016. LLNL-PRES-685853.
———. 2016c. “Ab initio Calculations of Ionic Conductivity in Lithium and Sodium Polyborate Solid Electrolytes.” Electrochemical Society PRiME Meeting, Honolulu, HI, October 2016. LLNL-PRES-704439.
———. 2016d. “Understanding and Improving Ionic Conductivity in Solid Electrolytes from ab initio Molecular Dynamics.” Mini-Symposium on Solid-state Batteries, Stanford, CA, November 2016. LLNL-PRES-691438.
———. 2017a. “Mechanisms and Motivations for Superionic Conductivity in Polyborane Solid Electrolytes from ab initio Molecular Dynamics.” American Chemical Society Meeting, San Francisco, CA, April 2017. LLNL-ABS-707706.
———. 2017b. “Understanding and Optimizing Ionic Conductivity in Polyborane Solid Electrolytes from ab initio Molecular Dynamics.” Electrochemical Society Meeting, New Orleans, LA, May 2017 (poster). LLNL-POST-732128.
———. 2017c. “Complex Dynamics in Metal Borohydrides: From Hydrogen Storage to Solid-State Batteries.” Hydrogen-Metal Systems Gordon Research Conference, Easton, MA, July 2017. LLNL-PRES-735183.
———. 2017e. “Graphene Derivatives for Energy Storage: Fundamental Insights from ab initio Simulations.” Telluride Workshop on Computational Materials Chemistry, Telluride, CO, August 2017. LLNL-PRES-736845.
———. 2017f. “Structural and Chemical Frustration in Solid Electrolytes from ab initio Molecular Dynamics.” World Conference on Solid Electrolytes for Advanced Applications, Pondicherry, India, September 2017. LLNL-PRES-728806.
———. 2017g. “Integrated Mesoscale Approach for Predicting Ionic Conductivity in Solid Electrolytes.” CalCharge Meeting, Livermore, CA, November 2017. LLNL-ABS-741263.
Ye, J., et al. 2015. “Universal Roles of Hydrogen in Electrochemical Performance of Graphene: High Rate Capacity and Atomistic Origins,” Scientific Reports 5: 16190. LLNL-JRNL-670804.