Brett Isselhardt (14-ERD-082)
Abstract
Our goal was to study the foundational science required to enable resonance ionization mass-spectrometry analysis for new applications. This work leveraged a capability that established a resonance ionization mass-spectrometry instrument at Livermore called LION (for laser ionization of neutrals). A high-transmission time-of-flight mass spectrometer coupled to six tunable titanium–sapphire laser cavities, LION enabled rapid development to improve the overall efficiency and reliability of analysis by resonance ionization mass spectrometry. Our first aim was to improve the efficiency of resonance ionization mass-spectrometry measurements by increasing the yield of neutral atoms of the analyte during the atomization event. The second aim was to improve the precision and accuracy of isotope-ratio measurements by optimizing the resonance ionization process. Our two primary achievements were improving the efficiency of resonance ionization mass spectrometry for elements like uranium by two orders of magnitude on real-world samples, while at the same time identifying potential paths for even higher efficiencies, and improving the quality of isotope-ratio measurements by optimizing the ionization schemes for uranium and plutonium via laser spectroscopy. Based on these enhancements of resonance ionization mass-spectrometry methodology, the LION instrument is now beginning to be applied to real-world samples in fields such as nuclear forensics, nonproliferation, and cosmochemistry.
Background and Research Objectives
Accurate isotope-ratio measurements are essential in fields such as geochemistry, cosmochemistry, nonproliferation, and nuclear forensics. Most current methods for quantifying isotope abundances require time-consuming sample preparation and separation chemistry. A few techniques, such as secondary-ion mass spectrometry and laser ablation inductively coupled plasma mass spectrometry, are capable of direct isotopic analysis but are limited by isobaric interferences. Resonance ionization mass spectrometry is a high-sensitivity, elementally selective, laser-based form of mass spectrometry that offers the potential to determine the isotopic composition of materials without sample preparation and isobaric interference. Resonance ionization uses pulsed laser light that is resonant with a electronic excited state to excite and eventually remove an electron from the atom or molecule to create positively charged ions, which are then collected and accelerated into a mass analyzer. The technique can analyze samples ranging from inclusions in meteorites to nuclear fallout.
Using element-specific laser ionization, this method has the potential to overcome some of the limitations of traditional mass-spectrometric analytical techniques. Resonance ionization mass spectrometry is not a recently developed concept.1 However, limitations in laser technology and reliability have severely limited its previous application to quantifying nuclear materials. Recent developments in laser systems technology and effort by Lawrence Livermore and Argonne national laboratories supported by the Department of Homeland Security National Technical Nuclear Forensics Center have enabled a new level of precision, accuracy, and reliability in quantifying uranium and plutonium isotope ratios in nuclear materials to better than 0.5%, the relative standard deviation from the mean.2,3 These techniques have been applied by our colleagues and others to obtain statistics-limited precision on measurements of barium isotope ratios in micrometer-sized silicon carbide grains where barium is present at the level of a few micrograms per gram.4
Analysis by resonance ionization mass spectrometry requires three steps: atomization of the sample, resonance ionization of the neutral atoms, and mass analysis and detection. First, atoms are desorbed from a sample surface using a pulsed laser or ion beam. A cloud of ions, molecules, and neutral atoms is generated above the sample containing all elements present in the desorbed volume. Ions generated during this process are electrostatically rejected by applying a large voltage pulse. Second, neutral atoms of the element of interest are selectively excited to an intermediate (resonant) electronic state by lasers tuned to a characteristic transition energy unique to that element. This process can be made more selective through use of two or more resonant excitation steps. The excited atoms are then photoionized from the intermediate state by an additional laser tuned to an auto-ionizing resonance (unbound metastable state) above the ionization potential for that element. Finally, these photo ions are accelerated into a mass spectrometer, mass analyzed, and detected. Chemical separation prior to analysis is not necessary, because all other elemental and molecular species are largely transparent to the laser photons at the wavelengths specific to the element of interest and remain undetected. Resonance ionization mass spectrometry has the potential to achieve high detection efficiency for most elements, but tends to lose efficiency for elements that form oxide molecules. We developed this method to enable the rapid, accurate determination of isotope abundances for uranium, plutonium, and other actinides and fission products without sample processing or dissolution chemistry.
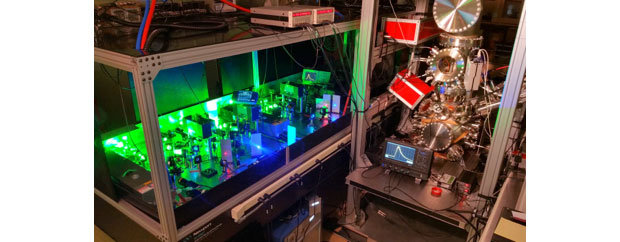
In 2015, the Laboratory completed construction of LION, a specially designed time-of-flight mass spectrometer coupled to resonance ionization lasers (see Figure 1). The ion optics are designed to efficiently extract ions from a region above the sample5 on the order of 1 cm3. The ions are accelerated to a voltage of 3 kV and follow a flight path of almost 4 m before being focused onto the detector. The instrument uses a fast (0.3-ns pulse width) microchannel plate detector to allow higher count rates. It is equipped with multiple methods for sample atomization, including primary ion sputtering with a liquid metal ion gun (gallium ion or gold ion), a gaseous ion beam gun (reactive or noble gasses), nanosecond-laser desorption, and a femtosecond-laser desorption system. The LION spectrometer has six tunable titanium–sapphire lasers available for ionization, to resonantly ionize multiple elements simultaneously.
Scientific Approach and Accomplishments
Measurements on uranium metal were performed to determine the total efficiency of the LION mass spectrometer. The measurements were defined as the number of atoms detected in a measurement divided by the number of atoms removed. A piece of uranium metal of natural isotopic composition was mounted into epoxy and polished to remove most of the uranium oxide surface composition. Once inside the vacuum chamber, a gold ion beam was used to sputter clean the metal, further reducing the amount of oxygen present on the surface. The ion beam was then focused to a smaller spot and measurements were made to detect uranium on the freshly cleaned surface, as shown in Figure 2. After analysis, the volume of the resultant crater was measured using a laser interferometer to determine the rate of removal of material from the sample.
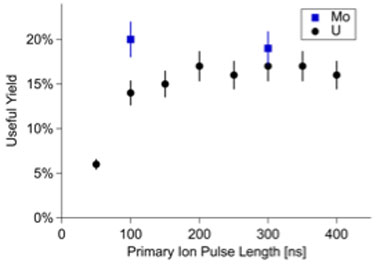
The useful yield was determined by dividing the number of ion counts by the calculated number of atoms removed for both uranium and molybdenum metal samples. The measured useful yield for molybdenum was 20 ± 2%, and 17 ±1 % for the sputter-cleaned uranium metal. Both values are consistent with instrument design criteria. This means that for every five atoms removed from a well-reduced sample we expect to detect one, an order-of-magnitude better than the detection rates typically achieved by secondary-ion mass spectrometry. Figure 3 shows the measured useful yield for several primary ion pulse lengths for both uranium and molybdenum metal samples.
A unique feature of the LION instrument is the availability of two different primary ion beam sources for analysis. The liquid metal ion beam is capable of analysis at fine spatial scales, while the gaseous ion beam (noble or reactive gases) is capable of physical and chemical modification of sample surfaces to improve the sensitivity of liquid metal ion beam measurements. This can be used as a pump–probe technique, in which one beam prepares the sample surface while the other analyzes it. The power of the dual beam approach is demonstrated in the physical reduction of a sample surface via preferential sputtering of oxygen over uranium in uranium dioxide. Using a single-beam approach, we have shown that pre-treatment of a uranium dioxide surface with gallium ions to deliver a calibrated ion dose (sufficient to remove ~50 nm of material) prior to fine probing with the same beam results in a substantial reduction of the sample surface, with the result that the useful yield during the analysis rises by more than an order of magnitude, to 2%. Pre-treatment with the same dose of argon ions is much more effective in reducing the sample surface because of the closer match between the argon and oxygen atomic masses, resulting in a further doubling of the useful yield during analysis with the gallium ion probe to 4%. This useful yield is better by at least two orders of magnitude compared to previous measurements.6 For comparison, competing spatially-resolved techniques such as secondary-ion mass spectrometry and femtosecond laser-ablation inductively coupled plasma mass spectrometry have useful yields on the order of 1 to 2% from oxide materials.7,8
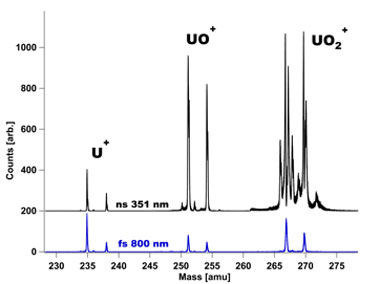
In addition to studying resonance ionization mass-spectrometry measurements by ion sputtering, an investigation of a novel femtosecond laser desorption approach was also explored to increase the neutral atom yield. It was contrasted with other commonly employed methods, namely a 25-kV gold ion beam and 351-nm nanosecond laser desorption. In these experiments, atomic uranium was ionized with nearly 100% efficiency by the resonance ionization scheme, while all other ions (e.g., uranium oxide ions and uranium dioxide ions) were produced by an inefficient non-resonant ionization process. A subset of the results is presented in Figure 4, with the 800-nm femtosecond laser observed to produce a marked decrease in molecular uranium oxides, relative to uranium atoms. This increase in the observed ratio of uranium to uranium oxides indicates a significant improvement in the resonance ionization mass-spectrometry detection efficiency of uranium from uranium oxide samples. Detailed experiments exploring the roles of wavelength, pulsewidth, and fluence on the uranium measurement efficiency in resonance ionization mass spectrometry represent the first successful application of femtosecond laser desorption to resonance ionization mass spectrometry.
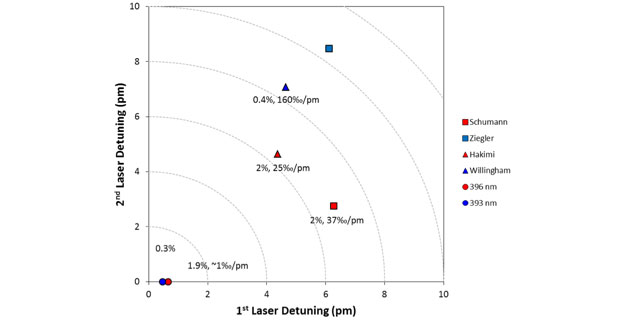
Figure 5 shows four three-color schemes and two one-color schemes. Three-color schemes are better at discriminating against other elements and generally easier to saturate. One-color schemes can be robust with respect to isotope shifts, but are usually difficult to saturate. Figure 5 plots the de-tunings for the first two steps of each scheme with the lasers tuned to the midpoints of the uranium-235 and uranium-238 resonances for each step. The detuning of the ionization step is irrelevant because we have modified the reported schemes by finding new auto-ionizing states that have the same wavelength dependence for all isotopes. In Figure 5, the further a scheme is from the origin, the less suited it should be for resonance ionization mass spectrometry. We measured the sensitivity of the schemes to changes in each laser wavelengths, given as the change in measured isotope ratio per picometer change in the laser wavelength (‰/pm). The figure also shows measured useful yields, with three of the six schemes achieving the maximum of 2% for uranium dioxide by gallium ion sputtering. The best scheme in Figure 5 is clearly 396-nm one-color. We evaluate it for mixed-actinide analysis, in which discrimination against plutonium will be important.
Impact on Mission
Our development of a state-of-the-art resonance ionization mass-spectrometry capability at Lawrence Livermore ensures continued leadership in the core competency of nuclear, chemical, and isotopic science and technology in support of national security, specifically nuclear threat reduction via nuclear detection and forensics. It also meets the goal of developing technological capabilities that advance scientific frontiers and address next-generation basic scientific issues related to the evolution of our solar system. The development of this spectrometric capability has provided for the conversion of one postdoctoral researcher, the hire of a mid-career scientist, and support to hire three postdoctoral researchers.
Conclusion
We have quantified the detection efficiency of the Livermore LION imass-spectrometer instrument to be around 20% for ideal samples and useful yields as high as 4% for uranium from oxide matrices, which represents at least two orders of magnitude improvement over previous resonance ionization mass-spectrometry measurements. We have demonstrated new laser desorption methods that indicate significant improvement in our efficiency to measure uranium in oxide materials. We have explored new ionization schemes for uranium and plutonium that may improve the simplicity and reproducibility of measured isotope ratios. We also demonstrated that the LION instrument is capable of accurately and reproducibly quantifying the uranium and plutonium isotope ratios to statistically limited precision as small as 0.4% using bulk reference materials that contain some isobaric interferences. Our study will enable the development of resonance ionization mass spectrometry for application to the fields of nuclear forensics, nonproliferation, and cosmochemistry. Based on this work, several new projects have been initiated with sponsors including the Department of Homeland Security's National Technical Nuclear Forensics Center, the National Nuclear Security Administration's Office of Defense Nuclear Nonproliferation, the National Aeronautics and Space Administration, and a joint project with the Naval Postgraduate School funded by the Defense Threat Reduction Agency's Basic Research Program.
References
- Hurst, G., et al., “Resonance ionization spectroscopy and one-atom detection." Rev. Mod. Phys. 51(4), 767 (1979). http://doi.org/10.1103/RevModPhys.51.767
- Isselhardt, B. H., et al., “Improving precision in resonance ionization mass spectrometry: Influence of laser bandwidth in uranium isotope ratio measurements." Anal. Chem. 83(7), 2469 (2011). http://doi.org/10.1021/ac102586v
- Isselhardt, B. H., et al., “Improved precision and accuracy in quantifying plutonium isotope ratios by RIMS.” J. Radioanal. Nucl. Chem. 307(3), 2487 (2016). LLNL-JRNL-674877. http://doi.org/10.1007/s10967-015-4393-x
- Liu, N.. et al., “Barium isotopic composition of mainstream silicon carbides from Murchison: Constraints for s-process nucleosynthesis in asymptotic giant branch stars." Astrophys. J. 786(1), 66 (2014). http://doi.org/10.1088/0004-637X/786/1/66
- Veryovkin, I. V., et al., ”A new time-of-flight instrument for quantitative surface analysis." Nucl. Instr. Meth. Phys. Res. B 219–220, 473 (2004). http://dx.doi.org/10.1016/j.nimb.2004.01.105
- Knight, K. B., et al., “Uranium resonance ionization mass spectrometry in natural U-silicate." Proc. Radiochim. Acta 1, 37 (2011). LLNL-JRNL-422855.
- Hervig, R. L., et al., "Useful ion yields for Cameca IMS 3f and 6f SIMS: Limits on quantitative analysis." Chem. Geol. 227(1–2), 83 (2006). http://dx.doi.org/10.1016/j.chemgeo.2005.09.008
- Ranebo, Y., et al., “Improved isotopic SIMS measurements of uranium particles for nuclear safeguard purposes." J. Anal. At. Spectrom. 24, 277 (2009). http://doi.org/10.1039/B810474C
Publications and Presentations
- Gates, S., et al., Resonance ionization mass spectrometry for actinide isotope measurements: Modeling and simulations. Intl. Conf. Radioanalytical and Nuclear Chemistry, Budapest, Hungary, Apr. 10–16, 2016. LLNL-ABS-678547.
- Isselhardt, B. H., M. R. Savina, and A. Kucher, Quantifying Pu and U isotope ratios in spent fuel samples by resonance ionization mass spectrometry. 9th Intl. Conf. Nuclear and Radiochemistry, Helsinki, Finland, Aug. 28–Sept. 2, 2016. LLNL-ABS-689517.
- Isselhardt, B. H., M. R. Savina, and A. Kucher, Recent progress in quantifying Pu isotope ratios by RIMS. SciX 2015, Reno, NV, Sept. 27–Oct. 2, 2015. LLNL-ABS-663575.
- Isselhardt, B. H., et al., Calculating relative ionization probabilities of uranium isotopes by resonance ionization mass spectrometry: Understanding the influence of laser induced bias on measured isotope ratios. (2015). LLNL-JRNL-648449.
- Isselhardt, B. H., et al., “Improved precision and accuracy in quantifying plutonium isotope ratios by RIMS.” J. Radioanal. Nucl. Chem. 307(3), 2487 (2016). LLNL-JRNL-674877. http://doi.org/10.1007/s10967-015-4393-x
- Isselhardt, B., et al., "Rate equation model of laser induced bias in uranium isotope ratios measured by resonance ionization mass spectrometry." J. Anal. At. Spectrom. 31, 666 (2016). LLNL-JRNL-648449. http://dx.doi.org/10.1039/c5ja00249d
- Savina, M., et al., Resonance ionization mass spectrometry for actinides: Plutonium analysis. Intl. Conf. Radioanalytical and Nuclear Chemistry, Budapest, Hungary, Apr. 10–16, 2016. LLNL-ABS-679207.