Salmaan Baxamusa (15-ERD-020)
Abstract
The goal of this project was to synthesize chemically stable and optically transparent plastics using chemical vapor deposition (CVD). Plasma-based CVD processes—which are the dominant technology for polymer CVD—result in polymers that absorb light and react with ambient oxygen and moisture. During this project, we discovered that the common source of both the optical absorption and unusual aging behavior of plasma CVD polymers is the plasma-based fragmentation of molecules during deposition. Considering this, we developed a non-plasma method for depositing polymer films of poly(divinylbenzene) (PDVB) that are optically transparent and chemically stable. Molecular design principles allowed us to design this film to have the high thermal and mechanical stability necessary for engineering applications. We measured the deposition stress in PDVB films to be extremely small, which enabled us to deposit arbitrarily thick layers, and explained the evolution of irreversible film stress upon thermal treatment. We also identified the primary source of PDVB aging, and developed a method to eliminate this aging pathway. These results gave us a fundamental understanding of the sources of ambient aging in CVD polymers, and allowed us to advance new processes and materials with properties superior to current technologies. We expect our new material to be an important option for plastic ablators used in future high energy density (HED) or Inertial Confinement Fusion (ICF) experiments, as well as a candidate for other protective coating technologies.
Background and Research Objectives
The most mature polymer CVD technology is plasma CVD in which the decomposition of an organic gas in a plasma discharge creates molecular fragments that then react to form a thin film at a substrate. This technology is used internationally for fabricating the plastic ablators used for high-energy-density (HED) and Inertial Confinement Fusion (ICF) experiments.
While plasma CVD polymers have outstanding thermal and mechanical properties, they chemically age through oxidation reactions. These long-term stability issues are a problem specifically in the fields of HED science and ICF science, where experiments are very sensitive to the chemical composition of the ablator. Moreover, these aging issues prevent plasma CVD polymers from being adopted in industrial applications despite their appealing thermal and mechanical properties. This problem has remained unsolved because there is limited control over the fragmentation that initiates plasma CVD polymerization. However, it is this fragmentation that leads to crosslinking, which results in outstanding thermal and mechanical stability.
Over the last 15 years, researchers have developed initiated chemical vapor deposition (iCVD), which limits molecular fragmentation to only a specific chemical termed the initiator. This enables the prediction of the properties of CVD polymers based on the molecular structure of the precursors, which potentially enables the creation of a “designer” CVD polymer that replicates the thermal and mechanical stability of plasma CVD polymers while displaying enhanced optical transparency and chemical stability.
The main objective of this project was to identify the dominant aging pathways in plasma CVD polymers, and then use iCVD to synthesize a polymer that would have the mechanical and thermal stability of plasma CVD polymers while displaying enhanced chemical stability.
Scientific Approach and Accomplishments
The major accomplishments of this project were as follows:
- discovery of a photo-oxidative aging pathway for plasma polymers that had its origins in the molecular fragmentation that occurs during plasma deposition
- development of a non-plasma CVD method to deposit PDVB—a chemically stable and optically transparent polymer that matches or exceeds plasma polymers in its optical, chemical, thermal, and mechanical properties
- first measurement of thin-film stress in iCVD films and a mechanism for the formation of irreversible thermal stresses—allowing us to deposit the thickest ever films of this type
- understanding of the molecular origins for spontaneous and photochemical aging in PDVB and development of a stabilization method based on a simple thermal treatment
- demonstration that high-Z dopants such as silicon can be incorporated into PDVB
The remainder of this report details these accomplishments. Additional details can be found in the publications listed at the end of this report.
Photo-oxidation as the dominant aging pathway for plasma CVD polymers
Plasma CVD polymers are known to exhibit unusual absorption of visible light. Instead of a sharp ultraviolet (UV) absorption edge, plasma polymers have unstructured broadband light absorption that extends far into the visible. Despite decades of study on the optical and structural characteristics of plasma polymers, little is known about their photochemistry.
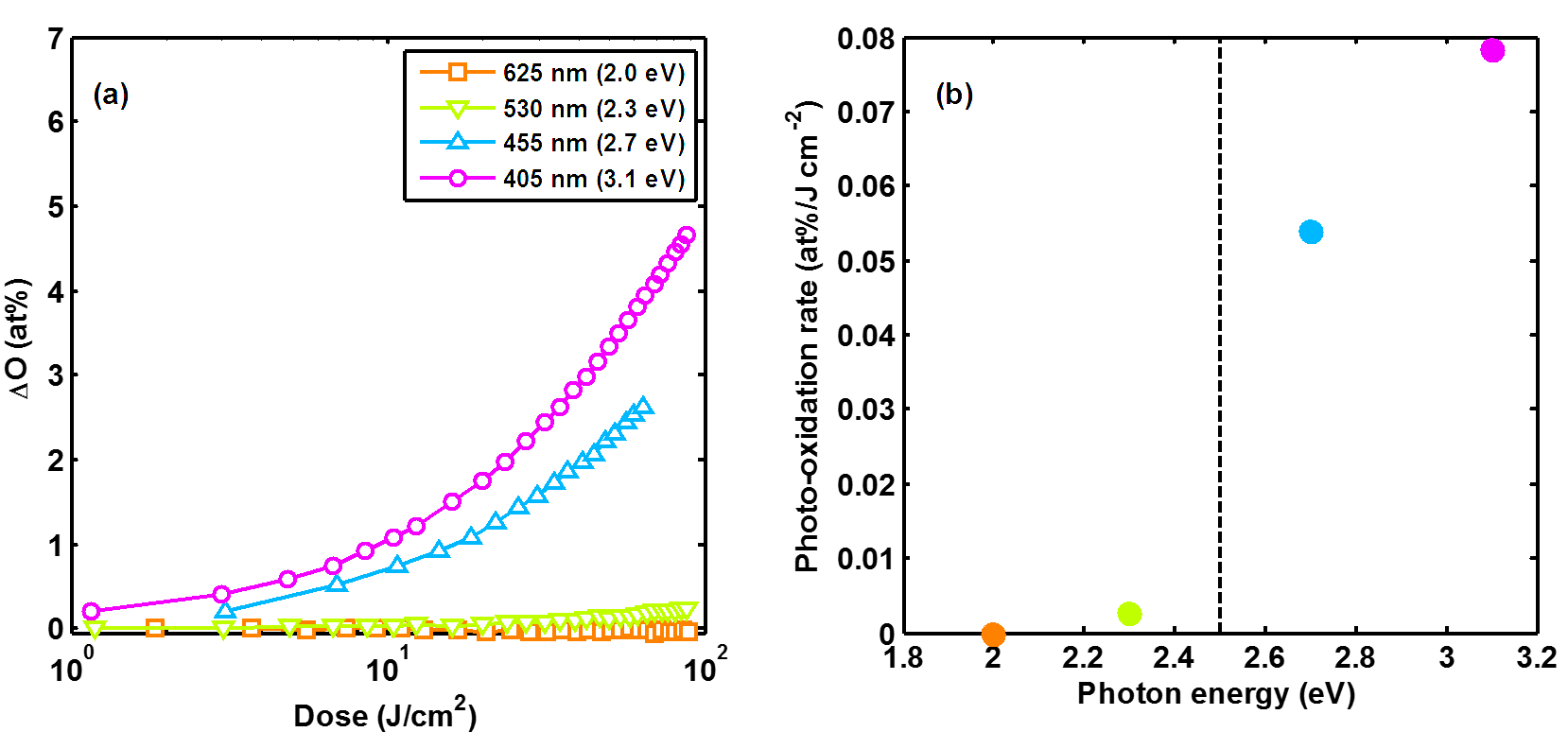
We studied the photo-oxidation of plasma polymers using in situ Fourier-transform infrared spectroscopy (FTIR). (See Figure 1.) To quantify oxygen content, the extinction coefficient of hydroxyl groups was measured by calibrating FTIR peak areas to mass gain. Plasma polymers photo-oxidized rapidly under visible light with a spectral dependence consistent with the measured optical gap of approximately 2.5 eV—it was faster for higher energy photons, but slower (though not eliminated) for lower energy photons. Plasma polymers stored in a dark ambient light oxidized far more slowly than those stored under fluorescent ambient light, which showed that the photochemical process was the dominant aging mechanism.
The optical absorption of plasma polymers was accompanied by quasi-continuum photoluminescence (QCPL), which is an optical phenomenon in materials with a high density of light-absorbing defects. Historically, these defects were believed to be residual radical species in the plasma polymer. However, we used a combination of electron paramagnetic resonance (EPR) and QCPL to show that photoactivity increased when the population of radicals decreased. Increased QCPL correlated to faster photo-oxidation. The defects responsible for optical absorption were therefore not unreacted free radicals. We have not unequivocally identified the nature of the defects, but they are likely small islands of sp2-bonded carbon.
Reducing plasma power during deposition reduced light absorption, decreased crosslinking, and slowed photo-oxidation. The combination of optical, structural, and photochemical characterization implicated plasma-based fragmentation as the origin of defects leading to photochemical instability. We therefore pursued the non-plasma, non-fragmentary iCVD method to synthesize CVD polymers without light-absorbing defects.
PDVB via iCVD matched or exceeded the properties of plasma CVD polymers
A commercial iCVD reactor was the key procurement for this project. In this reactor, liquid-phase precursors were vaporized and flowed as a gas into the reaction chamber held at reduced pressure between 100–200 mTorr. The deposition stage was cooled from between 10–15°C to promote adsorption of precursor vapors. An array of wire filaments was suspended above the deposition stage and heated to 250–350°C to selectively decompose initiator molecules to initiating radicals. The pressure, precursor flow rates, filament, and stage temperatures were controlled and recorded using data acquisition software.
Initiating radicals impinged upon surface-adsorbed monomers to start polymerization. Unlike plasma CVD, the monomer is not fragmented in iCVD. Therefore, the properties of the final material reflect the molecular structure of the monomer. We identified divinylbenzene DVB as a candidate monomer because it is a pure hydrocarbon with an aromatic structure and multiple points for crosslinking. Structurally, the synthesized PDVB was expected to be glassy, thermally stable, amorphous, and transparent.
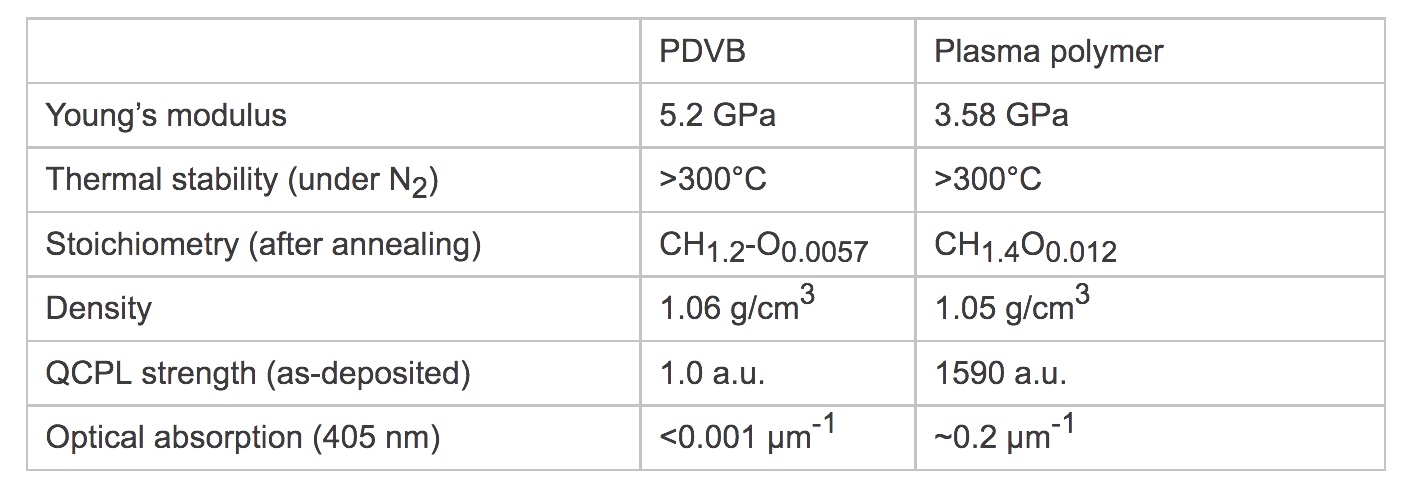
The iCVD synthesis of PDVB was previously reported, and we developed deposition conditions from literature reports, design calculations, and experimentation. Expanding on previous synthesis studies, we produced the first in-depth study of the material properties of PDVB. A summary of measured PDVB properties and a comparison to plasma polymer is shown in Table 1. Spectral verification of the polymer structure was made by FTIR—supporting the structure-property hypothesis of iCVD. Typical deposition rates were 0.6–1.0 μm per hour.
Crosslinking is central to the properties of PDVB. FTIR analysis showed that 44% of the incorporated monomer units were crosslinked through both vinyl groups. Despite the high density of crosslinks, we identified a glass transition (Tg) around 180°C via differential scanning calorimetry (DSC). Above Tg, PDVB maintains a remarkable degree of mechanical stability: coefficient of thermal expansion (CTE) increased by only 7% (192 ppm/K to 205 ppm/K, determined by ellipsometry) and Young’s modulus (E) dropped by a factor of about 3 times (5.2 GPa to 1.8 GPa, determined by nanoindentation). For a typical polymer, CTE and E may change by more than 2 times and 100 times respectively when heated above Tg.
The crosslinking also rendered PDVB infusible and insoluble—making iCVD the only practical route to cast smooth, conformal PDVB films. The surface roughness was independent of film thickness. The root mean square (RMS) roughness as measured by atomic force microscopy was 1–2 nanometers (nm) for films between 0.2–20 μm thick. The relatively high deposition pressure promoted conformal deposition on non-planar substrates.
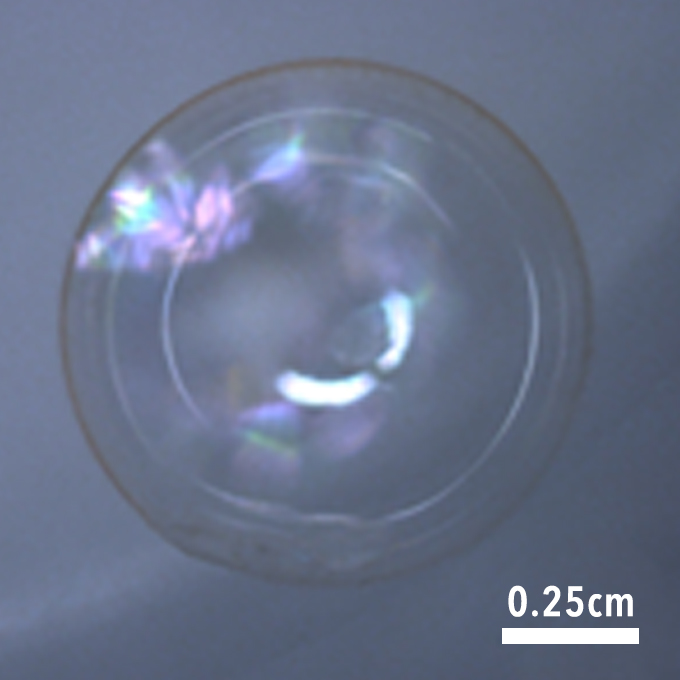
The combination of these properties—thermal, mechanical, surface, optical, and conformality—made PDVB compatible with ablator manufacturing. As conceptual proof, we deposited a 2 μm layer of PDVB on a spherical plastic mandrel and thermally decomposed the mandrel to produce a free-standing PDVB shell (Figure 2).
Intrinsic deposition stress is low and increases due to evaporation of unreacted monomer
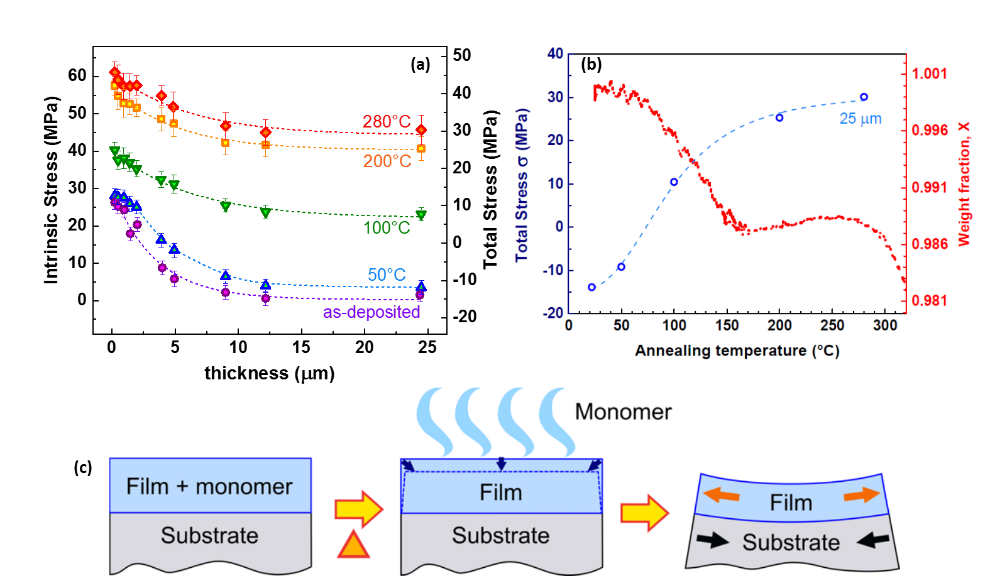
Both liquid and vapor approaches to crosslinked polymers can generate intrinsic film stress, which can lead to film cracking, delamination, or substrate damage. These stresses have never been measured in iCVD films. We used interferometry to make a curvature-based stress measurement as a function of thickness and thermal history—the latter to follow the development of irreversible thermal film stress. (See Figure 3a.)
PDVB begins growing under tensile stress, but beyond 5 μm, the net stress in the PDVB approaches zero. This implied that PDVB films could be grown arbitrarily thick. We deposited the thickest PDVB film yet reported at 25 μm. Stress was independent of whether deposition was done in a single step or multiple sequential steps.
Upon thermal cycling, an irreversible tensile stress of 45 MPa developed. Almost all of this increase occurred during thermal cycling below 200°C. The stress developed due to an irreversible 1.6% mass loss that was also completed by 200°C. Both the mass loss and the thermal evolution of film stress occurred only on the first heating cycle, and had an inflection point near 115°C; subsequent heating cycles showed reversible behavior. (See Figure 3b.) Mass spectrometry identified the lost material as DVB monomers. A volume contraction due to a 1.6% mass loss at constant density was consistent with a 45 MPa tensile stress. This mass-loss/volume-contraction mechanism is shown schematically in Figure 3c.
Aging pathways in PDVB can be passivated by treating unreacted vinyl groups
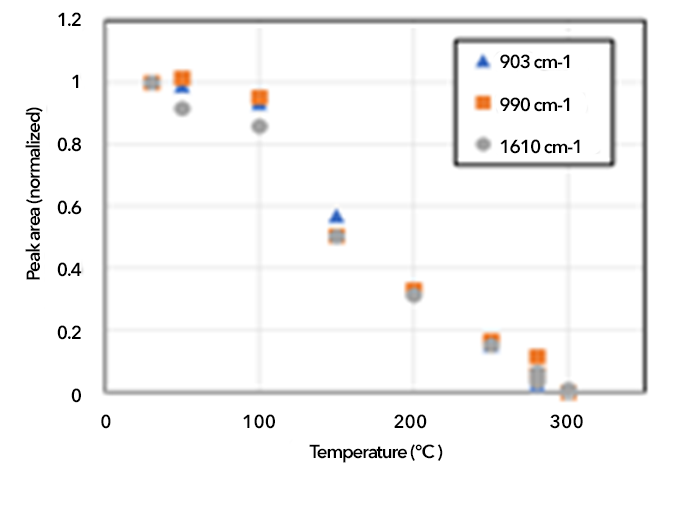
iCVD PDVB contained neither unreacted radicals nor photoactive defects, so it did not oxidize as readily as plasma polymers. However, unreacted pendant vinyl (C=C bonds) groups in PDVB could result in unwanted oxidation reactions. FTIR analysis showed that 44% of monomer units had formed crosslinks through the reaction of both vinyl groups; steric hindrance prevented additional crosslinking.
This aging pathway could be passivated by thermal annealing. Figure 4 shows the decrease in the vinyl content as a function of annealing temperature. DSC showed these vinyl groups decompose with a heat of reaction of -37 kJ per mol (exothermic)—roughly consistent with decomposing a sterically hindered vinyl group. Nearly all pendant vinyls were annealed following a 280°C treatment.
In as-deposited PDVB, unreacted vinyl was consumed as it reacted slowly with O2 to form carbonyl or hydroxyl. This vinyl-mediated oxidation was accelerated by ozone exposure and 365-nm light—or to a lesser degree 405-nm light—presumably due to the presence of reactive oxygen species.
After a thermal treatment to 280°C for four hours, PDVB did not react with ambient O2 and was stable under 405-nm illumination. However, it did oxidize somewhat under 365-nm illumination. This reaction was not traditional photo-oxidation. Rather, it was due to the generation of reactive oxygen species by the 365-nm light, which is consistent with the fact that annealed PDVB also oxidized when exposed to O3. These reactions occurred preferentially with pendant vinyl groups, so annealed PDVB was stabilized relative to as-deposited material. This behavior was a general property of polymers and not specific to PDVB (Lepro et al. 2017).
When stored under moist N2 (50% RH), PDVB neither absorbed nor reacted with water after three months.
iCVD PDVB can be doped with silicon
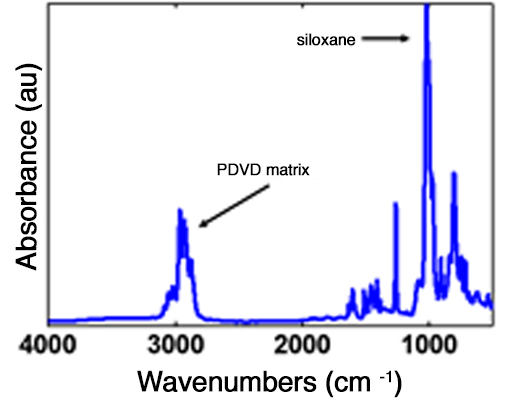
Ablators often needed to be doped with a high-Z material such as silicon (Si). We doped PDVB films with silicon by copolymerization with 1,3,5-trivinyltrimethylcyclotrisiloxane (V3D3), which has a similar vapor pressure to DVB. We verified copolymerization via the strong FTIR siloxane stretch at 1010 cm-1 seen in Figure 5. By altering relative flowrates, silicon could be incorporated in amounts ranging 2.0–3.7at.% as measured by Rutherford back-scattering (RBS) and correcting for the uncounted hydrogen atoms.
Si-doped PDVB was also decomposed in air to produce a clear, glassy residue of SiO2 to directly measure mole fractions of 90%/10% PDVB/V3D3. This corresponded to a silicon content of 1.3 at.%. The discrepancy between thermogravimetry and RBS is not yet understood.
We also attempted to copolymerize PDVB with vinyldimethylphenylsilane, a non-oxygen containing organosilane. The resulting polymer showed Si-Me and Si-phenyl stretches via FTIR, but dopant amounts were too low (<1at.%) to be quantified by RBS or electron dispersive spectroscopy.
Impact on Mission
Our work supports Lawrence Livermore's inertial fusion science and technology mission focus area and the NNSA goal of advancing the science, technology, and engineering competencies that are the foundation of the NNSA mission. We demonstrated that the plasma polymer used as the default plastic ablator in the international HED/ICF community has significant and previously unknown chemical instabilities. Programmatic adoption of these results lead to first-of-their-kind measurements of the impact of chemical heterogeneities on plastic ablators, and sparked a larger discussion on the future of plastic as an ablator platform. We also showed that chemically-stable plastic ablators are possible, and that programmatic investment in future development can lead to ablators that are chemically stable, optically transparent, and may have improved ablation performance.
The installation of an iCVD reactor generated expertise, publications, and presentations that have made LLNL a recognized world-leader in polymer CVD. Several new directions for iCVD polymers and composites for sensors, adhesives, and ultrastrong materials have been enabled by the new equipment, staff, and expertise. These applications are being pursued via funding streams within new and existing programs.
Conclusion
We have identified photo-oxidation as the dominant, but previously unknown aging mechanism in plasma CVD polymers. We also developed iCVD PDVB as an alternative that has equivalent (thermal and mechanical) or superior (chemical stability) properties and made the first in-depth study of its physical, chemical, and mechanical properties.
PDVB is being further developed for use as an ablator with programmatic support. Eventually, we hope to transfer this technology to a private partner who can manufacture PDVB-based HED and ICF ablators. We are also extending iCVD for potential use in ultrastrong composites and adhesives under new and existing funding streams.
References
Lepro, X., et al. 2017a. "Ultralow Stress, Thermally Stable Cross-Linked Polymer Films of Polydivinylbenzene (PDVB)." Langmuir, 33 (21): 5204–5212. doi:10.1021/acs.langmuir.7b01403.
Publications and Presentations
Baxamusa, S. 2016a. "Nuclear Fusion, Microbonding, and More: HWCVD Polymers at Lawrence Livermore." Presentation at the 9th International HWCVD Conference, Philadelphia, PA, September 9, 2016. LLNL-PRES-701011.
———. 2016b. "Photo-Oxidation Under Visible Light: The Strange Case of Plasma Polymers." Presentation at the Service Life Predictions of Polymeric Materials Conference, Santa Fe, NM, March 23, 2016. LLNL-PRES-684713.
———. 2017a. "PDVB: A CVD Approach to Alternative Plastic Ablators." Presentation at the Direct Drive Plastic Capsule Workshop, Livermore, CA, August 9, 2017. Title slightly different, LLNL-PRES- 736342, see attachment.
———. 2017b. "Polymers for Big Science: Free-Standing Films and Low-Stress CVD Materials at the National Ignition Facility." Presentation at the Massachusetts Institute of Technology Program in Polymers and Soft Matter Seminar Series, Cambridge, MA, May 3, 2017. LLNL-PRES-729684.
Baxamusa, S., et al. 2015a. "Photo-Oxidation of Polymers Synthesized by Plasma and Initiated CVD." Chemical Vapor Deposition, 21 (10-12): 267–274. doi:10.1002/cvde.201507173. Title slightly different “CVD” spelled out, LLNL-JRNL-669872.
———. 2015b. "Photo-Oxidation of Polymer-Like Amorphous Hydrogenated Carbon Under Visible Light Illumination." Polymer Degradation and Stability, 122: 133–138. doi:10.1016/j.polymdegradstab.2015.11.001.LLNL-JRNL-667678.
Laurence, T., et al. 2017. "Energy Transfer Networks: Quasicontinuum Photoluminescence Linked to High Densities of Defects." Physical Review Materials, 1 (6). doi:10.1103/physrevmaterials.1.065201. LLNL-JRNL-733134.
Lepro, X. 2016. "Stress and Stability of Poly(divinylbenzene) Films Produced by HWCVD." Presentation at the 9th International HWCVD Conference, Philadelphia, PA, September 6, 2017. LLNL-POST-701391 (2016).
Lepro, X., et al. 2017a. "Ultralow Stress, Thermally Stable Cross-Linked Polymer Films of Polydivinylbenzene (PDVB)." Langmuir, 33 (21): 5204–5212. doi:10.1021/acs.langmuir.7b01403. No document with this title.
———. 2017b. (forthcoming). "Thermal and Photostability of iCVD Polydivinylbenzene Films." LLNL-JRNL-740403-DRAFT, draft should not be referenced.