Holly Carlton | 18-FS-015
Overview
New developments in additive manufacturing (AM) enabled the design of complex artificial structures that are biocompatible and have properties similar to natural bone. The goal of our study was to identify promising ceramic-powder mixtures and demonstrate that a bio-inspired material for use as a bone replacement could be manufactured using laser sintering. We used a pulsed laser to sinter hydroxyapatite and tri-calcium phosphate powders together in different ratios optimized for physical properties. Although both ceramic powders are biocompatible, a balance of the two powders is desirable to achieve optimal formation of bone tissue around an implant. The laser parameter optimization part of this study was intended as a first step to transition this technology to other AM methods, like selective laser melting. We demonstrated that it was possible to sinter ceramic powder together with good control of the energy density. We used another powder mixture for comparison, mixing ceramic calcium phosphate powders with a polymer binder, polyether ether ketone (PEEK), to obtain different properties that could not be achieved with the ceramic powder alone. Due to the low melting temperature of PEEK, this mixture was challenging to sinter compared to the all-ceramic powder. We determined that there was a threshold value for energy density that would cause the powder and polymer mixture to bind successfully, but more research is needed. The next step of this study would be to perform similar pulsed-laser sintering on a larger volume of powder to create samples for mechanical tests and density measurements.
Background and Research Objectives
Finding suitable artificial replacement materials for non-healing bone defects is of extreme interest since such a large percentage of the population is affected by degenerative bone diseases; however, many of the candidate materials being considered have significant drawbacks. This is further complicated by the need to develop a material that is biocompatible, has a high level of strength and toughness, and has a low density to match natural bone’s structure and physical properties. Two new calcium-phosphate-based ceramics are promising: hydroxyapatite (HAP) and tri-calcium phosphate (TCP), both of which are biocompatible and have a composition and structure similar to that of natural bone. These materials must be manufactured by nonconventional means to create the desired shapes and properties, as well as to overcome processing challenges. Additive manufacturing (AM) methods can produce structures that have specific porosity and customized part geometry. The goal of this study was to combine new developments in processing ceramic powder with new manufacturing methods to address these challenges and optimize for physical properties at the relevant scales.
In addition to improving the manufacturing methods for HAP, this feasibility study identified several gaps in the understanding of these materials’ physical properties, specifically fracture toughness, surface finish, and pore connectivity, all of which need to be addressed in order to produce a successful bone replacement. The current approach to making novel bio-inspired materials is to process these materials using existing methods and then use conventional testing to assess their physical properties (Kivrak and Tas 1998, Taboas et al. 2003, Bose et al. 2013, Inzana et al. 2014). However, this approach does not address many of the main challenges. We determined that titanium alloys and HAP are biocompatible and adaptable for use with AM methods; they may be viable bone-replacement options because they induce bone formation. Researchers at Lawrence Livermore National Laboratory have studied titanium-alloy lattice structures in order to create materials with high strength and low density. Titanium alloys and HAP ceramics have very different properties: HAP behaves like a brittle ceramic with very little toughness, while titanium is higher in strength and can be heat treated to increase ductility. Although using AM methods with titanium alloy powder is an established process, titanium’s mechanical properties do not match those of natural bone and it does not induce new bone formation.
One challenge to working with HAP and TCP powder is figuring out how to sinter it because it is a hydrated phase that decomposes at approximately 1,200°C, which results in the collapse of the HAP structure. One way to address this is by introducing sintering agents such as polyether ether ketone (PEEK). Bioactive PEEK composites (e.g., PEEK and calcium phosphates) have been used to improve the bone–implant interface, but porosity control has been poor. Vaezi and Yang (2015) used extrusion free-forming AM and compressive molding. Producing solvent-based extrusion free-formed HAP scaffolds involves HAP paste, 3D printing and drying, debinding, and sintering. Other studies explored the design criteria for these types of scaffold materials (Tan et al. 2003 and 2005, Du et al. 2015). Design criteria for scaffold characteristics and properties include porosity, the surface-area-to-volume ratio, pore size, pore interconnectivity, structural strength and fracture toughness, shape, and biocompatibility. Another characteristic to consider is matching natural-bone density: The density of bone is 1.93.8 g/cc, while the density of titanium alloys is approximately 4–5 g/cc, and that of PEEK is approximately 1.3 g/cc. Shuai et al. (2013) concluded that combining TCP and HAP ceramic powders in a weight ratio of 30:70 TCP to HAP, respectively, was optimal for mechanical properties. We used the results of that study as a starting point for our selection of powder, weight ratio, and laser-energy density. Another study (Tan et al. 2003) investigated blending HAP and PEEK powder using pure PEEK to determine the sintering temperature. That study informed our selection of the PEEK and ceramic powder mixture.
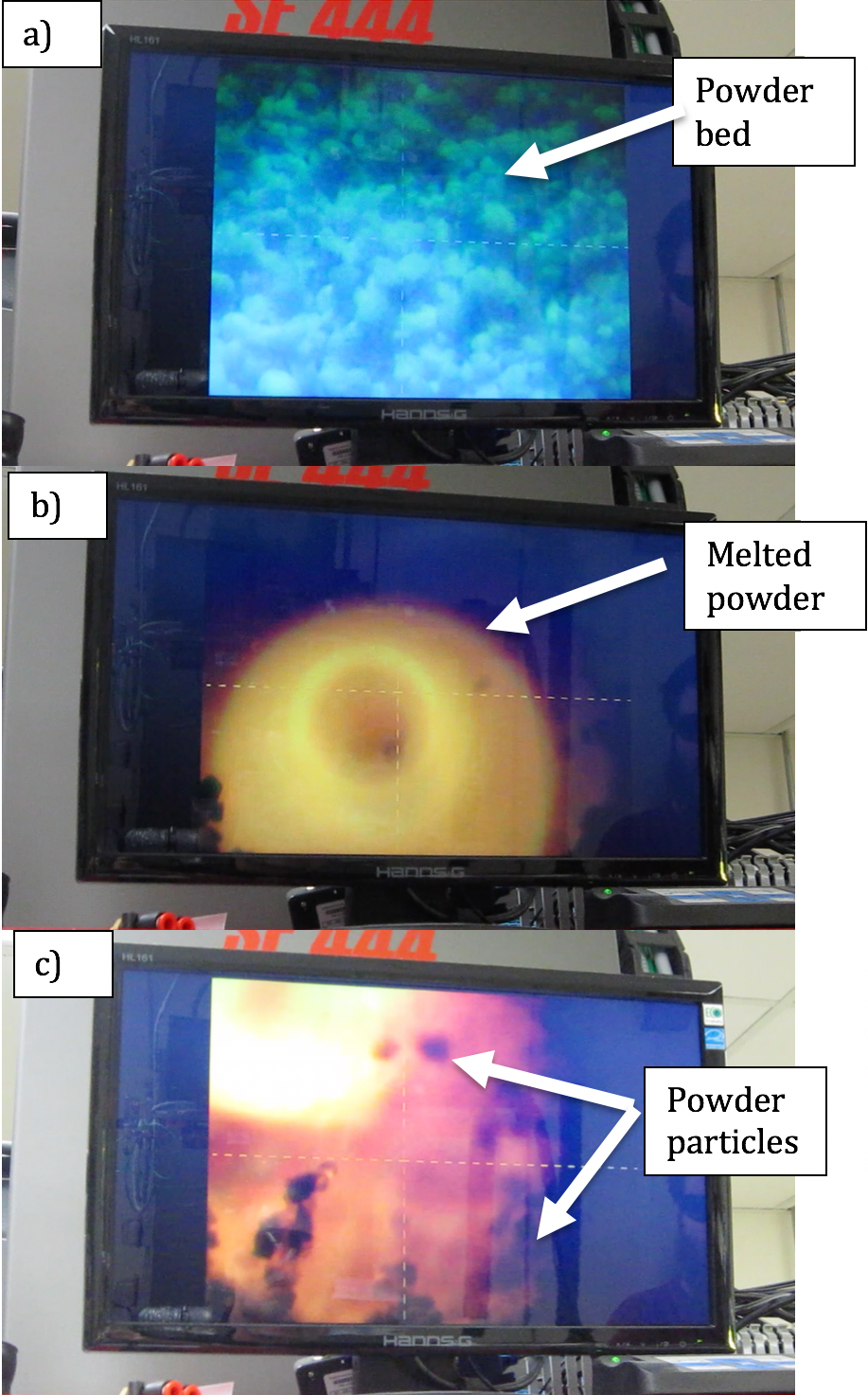
For this study, a pulsed laser was selected for sintering the powders; scanning electron microscopy and energy-dispersive spectroscopy techniques were used to evaluate the sintered ceramic samples (see figure below).
Impact on Mission
This feasibility study supports the NNSA goal to advance the science, technology, and engineering competencies that are the foundation of the NNSA mission. Specifically, this research enhances the Laboratory’s core competencies in bioscience, bioengineering, and advanced materials and manufacturing.
Conclusion
The most significant accomplishment of this feasibility study was the successful sintering of the mixture of powders after optimizing the pulsed-laser setup. For future studies, selective laser melting or other AM methods may be used to build up three-dimensional structures. The next step would be to conduct research aimed at sintering larger samples in order to get more characterization work done. Other future work may include (1) verifying cell regeneration on the sintered ceramic material and (2) transferring the results of the laser-sintering process to selective laser melting to try making a larger, porous, bone-like material. Overall, the process of sintering ceramic powders together shows promise as a viable bone-replacement option. Based on the needs identified in the literature, we recommend that future work focus on powder characterization, selective laser melting, fracture toughness testing, advanced in situ testing, porosity measurements, and biocompatibility assessments.
References
Bose, S., et al. 2013. "Bone Tissue Engineering Using 3D Printing." Materials Today 16(12): 496–504. doi: 10.1016/j.mattod.2013.11.017.
Du, Y., et al. 2015. "Microsphere-Based Selective Laser Sintering for Building Macroporous Bone Scaffolds with Controlled Microstructure and Excellent Biocompatibility." Colloids and Surfaces B: Biointerfaces 135 (Supplement C): 81–89. doi: 10.1016/j.colsurfb.2015.06.074.
Inzana, J. A., et al. 2014. "3D Printing of Composite Calcium Phosphate and Collagen Scaffolds for Bone Regeneration." Biomaterials 35(13): 4026–4034. doi: 10.1016/j.biomaterials.2014.01.064.
Kivrak, N. and A. C. Tas. 1998. "Synthesis of Calcium Hydroxyapatite-Tricalcium Phosphate (HA-TCP) Composite Bioceramic Powders and Their Sintering Behavior." Journal of the American Ceramic Society 81(9): 2245–2252. doi: 10.1111/j.1151-2916.1998.tb02618.x.
Shuai, C., et al. 2013. "Optimization of TCP/HAP Ratio for Better Properties of Calcium Phosphate Scaffold via Selective Laser Sintering." Materials Characterization 77 (Supplement C): 23–31. doi: 10.1016/j.matchar.2012.12.009.
Taboas, J. M., et al. 2003. "Indirect Solid Free Form Fabrication of Local and Global Porous, Biomimetic and Composite 3D Polymer-Ceramic Scaffolds." Biomaterials 24(1): 181–194. doi: 10.1016/S0142-9612(02)00276-4.
Tan, K. H., et al. 2003. "Scaffold Development Using Selective Laser Sintering of Polyetheretherketone–Hydroxyapatite Biocomposite Blends." Biomaterials 24(18): 3115–3123. doi: 10.1016/S0142-9612(03)00131-5.
——— . 2005. "Fabrication and Characterization of Three-Dimensional Poly Ether Ketone/Hydroxyapatite Biocomposite Scaffolds Using Laser Sintering." Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine 219(3): 183–194. doi: 10.1243/095441105X9345.
Vaezi, M. and S. F. Yang. 2015. "A Novel Bioactive PEEK/HA Composite with Controlled 3D Interconnected HA Network." International Journal of Bioprinting 1: 66–76. doi: 10.18063/IJB.2015.01.004.