Alan DeHope (15-ERD-036)
Abstract
The development of high explosive ingredients with detonation velocities and pressures greater than the current state-of-the-art materials has been stymied in recent years due to limitations in the achievable densities of organic explosives. Metal-containing explosives have been used with great success in many applications, including advanced blast munitions and primary initiating explosives. The high energy density of metals makes them attractive fuel ingredients for post-detonation combustion effects. However, metalized explosive mixtures fail to enhance detonation velocity and pressures due to the inability of the metal fuel to fully react to oxidized products on time scales similar to the organic explosive components of the mixture. Thermodynamic-equilibrium calculations predict that metal fuels can contribute to increased detonation velocities and pressures if all the metal fuel reacts into detonation products on the detonation timescale. This report describes our efforts to prepare molecules with metal fuel and organic oxidizer intramolecularly bound, to serve as explosive ingredients. An intramolecular fuel-oxidizer arrangement eliminates the diffusion limits typically encountered in heterogeneous compositions of metal fuel and oxidizer.
Background and Research Objectives
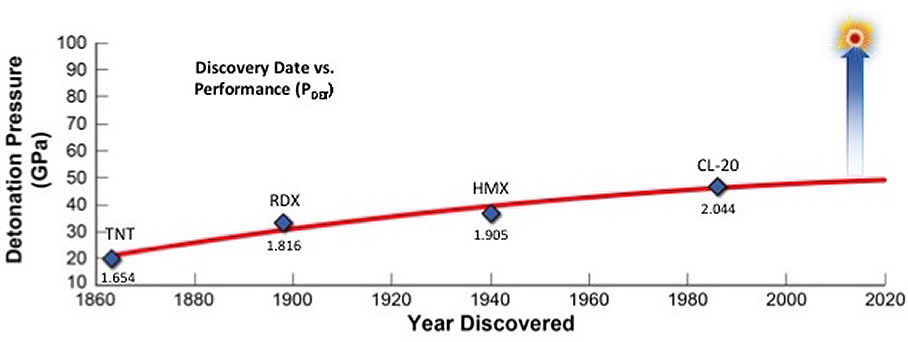
High explosives (HEs) play an integral role in national defense as ingredients in conventional munitions as well as nuclear weapons. The development of better-performing HEs has been hampered by the limitations of conventional organic explosives and their formulations. The graph in Figure 1 is a plot of the discovery year versus the detonation pressure (derived from the Cheetah thermochemical code [Bastea et al. 2015]), an important performance metric for four military HEs (TNT, RDX, HMX, and CL-20), and it illustrates the situation. The presence of an asymptote at approximately 50 gigapascals (GPa) of detonation pressure is evident. Significantly improving HE performance is an unmet scientific challenge that has persisted for decades.
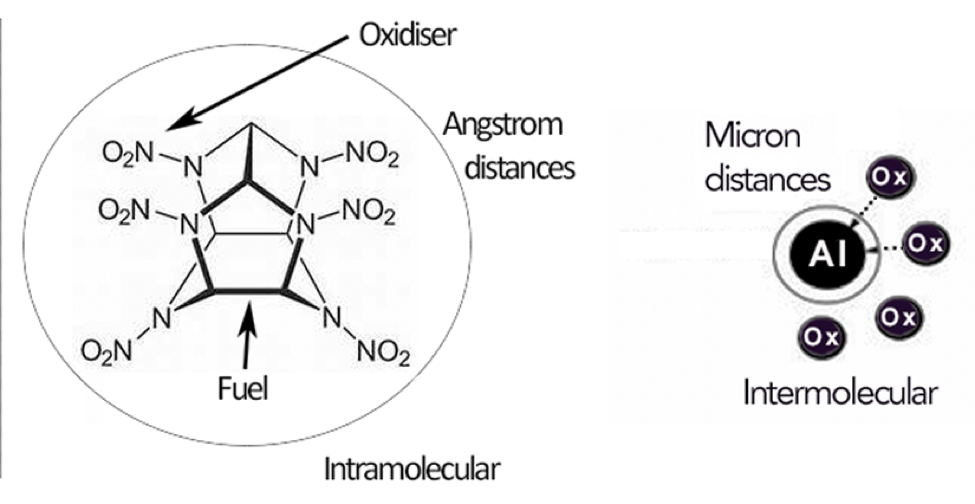
The goal of our research was to realize improvements in detonation performance by exploiting the high densities and large amounts of stored chemical energy available in metal fuels (Dreizin 2009) by combining a metal atom and organic oxidizer in the same molecule. Mixing metal fuels and organic explosives is not a new development: Tritonal, a mixture of trinitrotoluene (TNT) and aluminum metal dates back to the late 1800s. But to date, formulation and ingredient technology has not progressed enough to a point where the stored energy of the metal fuel contributes to the actual detonation. These heterogeneous mixtures of metal and HE are typified by their post-detonation chemistry. While very useful for many applications, they do not result in an increase in the actual detonation pressure and velocity. Oxidizer must travel long distances to react will all the metal fuel, inhibiting the release of all the combustion energy on the detonation timescale (Figure 2). The objective of this project was to create a path for extracting the stored energy of metal fuel in detonation via synthesis of complexes of metals and ligands, which themselves contained oxidizing functional groups.
Scientific Approach and Accomplishments
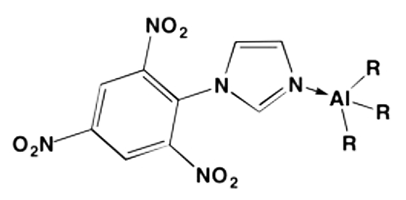
The approach taken to release metal combustion energy in detonation was to utilize electron donor ligands as oxidizer carriers on metal atoms. N-heterocyclic carbenes (NHCs) are a well established class of ligands for transition metal catalysts and can be prepared with a multitude of functional groups (Herrmann, et al. 1995; Bourissou et al. 2000; Hopkinson et al. 2014). Other electron-donating ligands, such as azoles, were also investigated. Aryl imidazole complexes to alkyl aluminum have been reported (Wu et al. 2015). Picryl imidazole (de Rossi and Buján 1981) was prepared and reacted in toluene with a solution of trimethylaluminum, affording a brown solid that was characterized by nuclear magnetic resonance (NMR) spectroscopy. The proposed structure is depicted in Figure 3.
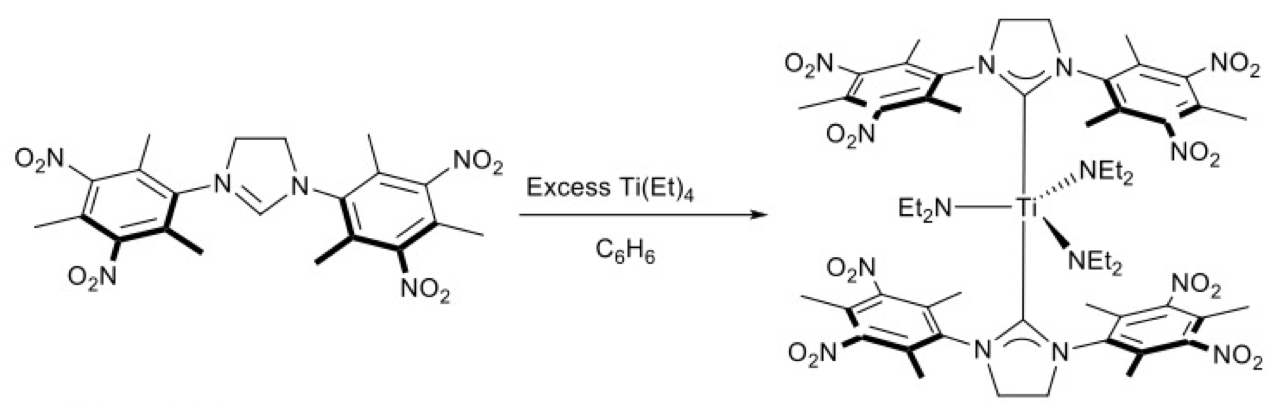
A titanium complex was also prepared utilizing an amine elimination strategy (Shukla et al. 2004) and its believed structure is depicted in Figure 4. The tetrafluoroborate salt of the nitrated dihydroimidazolium ligand precursor (Vorfalt et al. 2009) was prepared and reacted with tetrakis(diethylamido)titanium in benzene. The formation of the complex was monitored and characterized by NMR. Single crystals suitable for x-ray diffraction studies were not obtained.
Impact on Mission
Our research supports the Laboratory's core competency in advanced materials and manufacturing by addressing a key technical challenge in the field of energetic materials, utilizing the high energy density of metal in detonation. The progress we made in preparing a new type of ingredient that could lead to advances in detonation science is also relevant to the Laboratory's strategic focus area in stockpile stewardship science. The capability to handle air-sensitive explosive ingredients and their precursors has been established at the Laboratory and can benefit future materials-synthesis projects.
Conclusion
As a result of our research, a material type has been prepared that contains a metal atom and an energetic ligand intramolecularly bound. The next step forward for this research is to test the detonation performance of the produced metal complexes in a formulation. Results from these performance tests can be compared to the thermodynamic-equilibrium calculations to ensure that all the of available chemical energy is released on the detonation timescale. Positive results should lead to optimization of the metal complex's properties, such as chemical stability, oxygen balance, and density.
References
Bastea, S., et al. 2015. Cheetah 8.0 (energetic materials code). LLNL-CODE-677327.
Bourissou, D., et al. 2000. "Stable Carbenes." Chemical Reviews 100 (1): 39–92. doi: 10.1021/cr940472u.
de Rossi, R. H., and E. I. Buján. 1981. "Buffer Catalysis in the Hydrolysis of Picrylmidazole." Journal of the American Chemical Society 103 (6): 1533–1540. doi: 10.1021.ja00396a040.
Dreizin, E. L. 2009. "Metal-Based Reactive Nanomaterials." Progress in Energy and Combustion Science 35 (2): 141–167. doi: 10.1016/j.pecs.2008.09.001.
Herrmann, W. A., et al. 1995. "Metal Complexes of N-Heterocyclic Carbenes—A New Structural Principle for Catalysts in Homogeneous Catalysis." Angewandte Chemie International Edition 34 (21): 2371–2374. doi: 10.1002/anie.199523711.
Hopkinson, M. N., et al. 2014. "An Overview of N-Heterocyclic Carbenes." Nature 510: 485–496. doi: 10.1038/nature13384.
Wu, M., et al. 2015. "Synthesis, Structural Studies and Ligand Influence on the Stability of Aryl-NHC Stabilised Trimethylalumminium Complexes." Dalton Transactions 44: 15166–15174. doi: 10.1039/C5DT00079C.