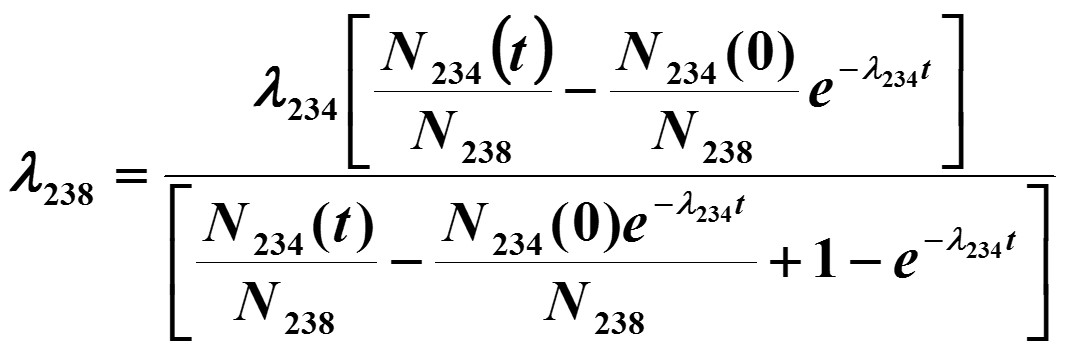Tashi Parsons-Davis (16-LW-053)
Abstract
Radioisotopic dating techniques are highly valuable tools for understanding the history of physical and chemical processes in materials related to planetary sciences and nuclear forensics and rely on accurate knowledge of decay constants and their uncertainties. The decay constants of U-238 and U-235 are particularly important to earth science, and often the measured values with lowest reported uncertainties are applied, though they have not been independently verified with similar precision. New direct measurements of the decay constants of U-238, Th-234, U-235, and U-234 were completed, using a range of approaches. An overarching goal of the project was to ensure the quality of results, including metrological traceability, to facilitate implementation across diverse disciplines. This report presents preliminary results of these measurements, as a few final measurements and calculations are still in progress.
The U-238 decay constant was determined via measurement of Th-234 ingrowth in chemically purified, isotopically enriched U-238 solutions, by quantitative extraction of the Th, the abundance of which was determined mass-spectrometrically after complete decay to U-234. The method relied on a starting material with low 234 content, careful yield tracing, and accurate measurements using the isotope-dilution (ID) method with multi-collector inductively-coupled plasma mass spectrometry (MC-ICP-MS). The experiment resulted in the decay constant 4.934 ± 0.011 E-18 inverse seconds, 0.37 ± 0.24 percent larger than that previously reported from alpha counting, with relative uncertainty of 0.23% 2-sigma. To reduce the uncertainty propagated from that of the Th-234 decay constant, Th-234 decay curves were measured with both a windowless gas-proportional counter and a high-purity Ge (HPGe) gamma detector. Preliminary results agree with evaluated literature values, which were used in the U-238 decay constant calculation. For the measurement of U-235 and U-234 decay constants, isotopically enriched solutions were chemically purified and analyzed for U concentration and isotopic composition by MC-ICP-MS. Counting sources were prepared with a known quantity of U and the absolute alpha activity was measured by alpha-gamma coincidence and low-geometry alpha counting. The U-235 activities measured by alpha-gamma coincidence counting are concordant with expected values, but higher-purity material and additional experiments are needed to reach the desired precision. Measurements of U-234 sources with the low geometry counter are still in progress, but initial results suggest a U-234 decay constant of 9.002 ± 0.030 E-14 inverse seconds, agreeing within uncertainty with previous alpha-counting results but slightly discordant with evaluations based on geological samples. The gamma emissions from the U-234 sources were measured with planar HPGe detectors to improve the gamma branching ratio precision, which is important for safeguards applications. Preliminary results are branching ratios of 0.1222 ± 0.0054 percent and 0.03529 ± 0.0012 percent for the 53.2 and 120.9 KeV gammas emissions, respectively. These results agree with evaluated data and provide greater than five times improvement in precision for the 120.9 keV line.
Background and Research Objectives
The application of chronology in the field of nuclear forensics has been one of the most exciting developments of recent years (Bibby et al. 2010). Similarly, a recent report on the current state of geochronology (Harrison et al. 2015) highlights the growth of high-precision U–Pb age-dating techniques, and their potential to help answer important questions regarding the evolution of life on Earth, as well as the interconnections between the Earth’s geochemical systems and climate variability. These reports emphasize the importance of accurate and precise decay constants (Λ) for application of U-series, U–Pb, and Pb–Pb dating to nuclear materials and the geological timescale. With the development of modern, advanced measurement techniques, the precision and accuracy, and therefore utility, of such ages is now limited by knowledge of the decay constants involved. As noted by Ludwig (2003), the uncertainty of a date is as important as the date itself.
The decay constants for the alpha decay of U-238 and U-235 are particularly important to earth science, as they not only limit the accuracy and precision of U–Pb and Pb–Pb dating, but also serve as calibration benchmarks for other decay schemes used in chronology (e.g., Renne et al. 2010). The uranium decay rates have been determined by defined-geometry alpha counting with high internal precision (~0.13% 2 sigma, Jaffey et al. 1971), but have not been independently verified with similar precision. Thus, much of the collective understanding of Earth history is tied to the accuracy of a single published, and unverified measurement. This situation is far from satisfactory, particularly because no consideration of systematic uncertainties or biases was incorporated into the errors reported by Jaffey et al. There is some concern in the geochronology community that one or both of these commonly used uncertainties was underestimated, as systematic differences are now observed between Pb-207/Pb-206, Pb-207/U-235, and Pb-206/U-238 dates (Schoene et al., 2006). Evaluations from several leading scientific organizations have called for new measurements of these important decay constants (Harrison et al. 2015; Schoen et al., 2003; Villa et al. 2016).
Our team of Livermore radiochemists, physicists, and nuclear-material experts, in collaboration with the Berkeley Geochronology Center, was well-positioned to take on these challenging measurements, with the availability of U-238, 235, and 234 samples of high isotopic purity and a suite of optimized uranium analytical techniques that have been developed for nuclear forensics. Two objectives in this project were to measure the decay constants of U-238 and of U-235, with sufficient precision for evaluation of the Jaffey 1971 values. We took a unique approach to the Λ-U-238 measurement that quantified ingrowth of Th-234 into purified aliquots of highly-enriched U-238, using ID-MC-ICP-MS. The method is independent of previous alpha-counting results, but relies on the accuracy of the Th-234 decay constant, which was last measured in 1948 (Knight and Macklin 1948), and the need for new measurements has been identified (Luca 2010). Thus, an auxiliary objective was to measure the Th-234 decay constant with less than 0.1% relative uncertainty.
The last two objectives of the project were to remeasure the U-234 decay constant with less than 0.2% relative uncertainty, and the gamma branching ratios to improve precision on the key 120.9 keV line at least five-fold relative to evaluated literature. The U-234 decay constant uncertainty can have a substantial impact on U-series and U–Th dating techniques. These chronometers apply to timescales from less than a year to approximately 600,000 years. U-series and Th-based chronometers have the capacity to provide a high-resolution paleoclimate record, leading to better understanding of global climate change. Th-230/U-234 age-dating of uranium materials also has important application in nuclear forensics, where 10 to 20% of the uncertainty comes from U-234 decay constant uncertainty. The weighted average of the two best direct measurements of the U-234 decay constant (De Bievre et al. 1971 and Lounsbury et al. 1971) has a two-sigma uncertainty of nearly 1%, leading to unacceptably large uncertainties in ages greater than 350 ka (Cheng et al. 2000). More precise values have been determined indirectly by measuring U-234 to U-238 isotope ratios in samples assumed to be closed systems (Cheng et al. 2000; 2013), but these values differ from directly measured values and rely on untested assumptions (Villa et al. 2016).
Two of the five objectives above were completely met (U-234 branching ratios and Th-234 decay constant), and three were partially met (U-234, U-235, and U-238 decay constants), meaning the measurements were achieved, but with relative uncertainties slightly higher than desired. It is worthwhile to note that much greater attention has been paid in recent years to realistic and comprehensive uncertainty assessments in radionuclide metrology and half-life measurements (Pommé 2015a). We aimed for diligence and metrological traceability, which should render our results valuable to the scientific community, even without meeting our original precision goals.
Scientific Approach and Accomplishments
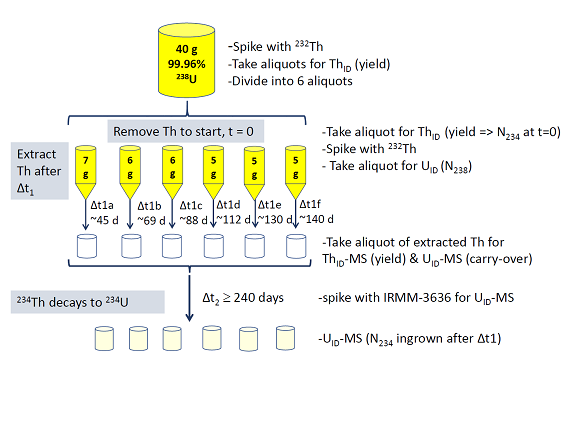
Determination of U-238 decay constant with novel ingrowth method
Our approach to determining the U-238 decay constant, summarized in Figure 1, has not been previously attempted, and was possible only because of our stock of “Q-metal” uranium, which is greater than 99.96% U-238 and contains only 3 ppm U-234. We measured ingrowth of the Th-234 daughter into purified aliquots of Q-metal in solution (5 to 7 g U in 4 M nitric and 0.005 M hydrofluoric acids), after different lengths of time (Δt1) between nominally 45 and 140 days. All quantitative measurements were obtained using ID-MC-ICP-MS, and the ingrown Th was extracted after Δt1 with careful yield tracing, then measured as U-234 after at least 10 half-lives (Δt2). The U solutions were spiked with Th-232 for yield tracing in both extractions, which were achieved by lanthanum fluoride co-precipitation, and, for the second extraction (after Δt1), followed by conversion to the HCl matrix and anion exchange columns. All chemistry included process blanks that were measured with samples. Sub-aliquots of the uranium solutions were removed for analysis before and after the initial purification and again before the second extraction, and sub-aliquots of the extracted Th after the second extraction were taken for analysis. Throughout the experiment, solution homogeneity was extremely important, and combinations of gentle heat (between 70 and 110 degrees Celsius) and sonication were used to homogenize solutions. Thorium-isotope dilution (ThID) was conducted using a Th-229 spike solution that was calibrated against a Th-230 standard reference material from the National Institute of Standards and Technology (NIST SRM 4342A), with extraction yields determined by bracketing to avoid any systematic uncertainty in spike concentration. Uranium-isotope dilution was conducted using a U-233 and U-236 double spike from the Institute for Reference Materials and Measurements (IRMM-3636). A Nu Plasma Model 1 MC-ICP-MS (Nu Instruments) was used for all measurements, with Faraday detectors. All acids used were either ultra-pure (Seastar) or trace-metal grade (Sigma-Aldrich), and all solution vessels were cleaned and acid-leached with perfluoroalkoxy alkane (PFA, Savillex). All weights were averages of three or more measurements with calibrated Mettler–Toledo or Sartorius analytical balances, checked using a common certified calibration weight set. The technical details of each step of the Th ingrowth experiment are too lengthy for this report but are described thoroughly in a journal article that is currently in preparation.
The half-life of U-238 is so long that the number of U-238 atoms in each aliquot (N238) was effectively constant during the experimental timescale, and the Bateman equation was rearranged to solve for the U-238 decay constant using experimentally measured quantities from each aliquot (Equation 1), where t is the aliquot ingrowth time (Δt1 above), λ238 is the U-238 decay constant, λ234 is the Th-234 decay constant, N238 is the number of U-238 atoms in the aliquot (the same at t = 0 and t, within measurement capabilities), N234(0) is the number of Th-234 atoms present in the aliquot at t = 0 (after initial purification) and N234(t) is the number of Th-234 atoms present in the aliquot at time = t (measured at U-234 after Δt2).
Equation 1.
The measured values and analytical uncertainties that contributed to each variable in Equation 1 were represented as vectors of pseudorandom numbers drawn from appropriate Normal distributions in a MATLAB code with all numerical constraints appropriately defined, and the equation was solved using a Monte Carlo sampling method (Metropolis and Ulam 1949) with at least 1E7 iterations. This was the most convenient way to account for the effect of covariances, such as the same spike being used for U-ID measurements.
The ingrowth times and their uncertainties were calculated by Monte Carlo sampling, considering the recorded times and approximate U and Th distributions (estimated with uncertainties based on gamma-count data, process knowledge, and measured overall yields) at each stage of both chemical separations. Decay and ingrowth in each step were calculated using the evaluated literature Th-234 decay constant and uncertainty (Luca 2010) and the U-238 decay constant iteratively, with the result of this work included. The calculated ingrowth times were always shorter than the nominal ingrowth times because the extracted Th was decaying during purification, and this effect increased as the ingrowth times approached secular equilibrium. The evaluated Th-234 decay constant (Luca 2010) and its uncertainty was also used in the calculation of λ238. Our preliminary measurements of the Th-234 half-life support this value (see next section). N238 was determined from average U-ID data collected over three different mass-spectrometry campaigns, on four total secondary dilutions from each of two duplicate dilutions of duplicate aliquots taken from the U-238 solution before the second extraction. The reproducibility of these measurements to determine the primary U concentration turned out to be the largest contributor to propagated uncertainties. N234(0) in each aliquot was determined by assuming secular equilibrium prior to the initial extraction (the master solution had last been purified three years before), and the yields of the first extraction were determined by Th-ID, in which 229/232 ratios were measured in Th-229 spiked aliquots of the U solution before and after the first extraction, which were bracketed in the ICP-MS measurement. The yield of the second extraction was measured using average Th-ID data of duplicate aliquots of the U solution before the second extraction and duplicate aliquots of the extracted Th, which were also bracketed. This yield was important for adjusting the value of N234(t), which was measured by U-ID after Th-234 had decayed to U-234 (extracted Th was stored in solution and spiked with a standardized dilution of IRMM-3636 during Δt2, allowing four months for equilibration). The final measurements quantified between 1.48E11 and 1.98E11 atoms in a low-blank environment, using the 235/238 ratios to strip 234 contributions from carried-over Q-metal and laboratory background.
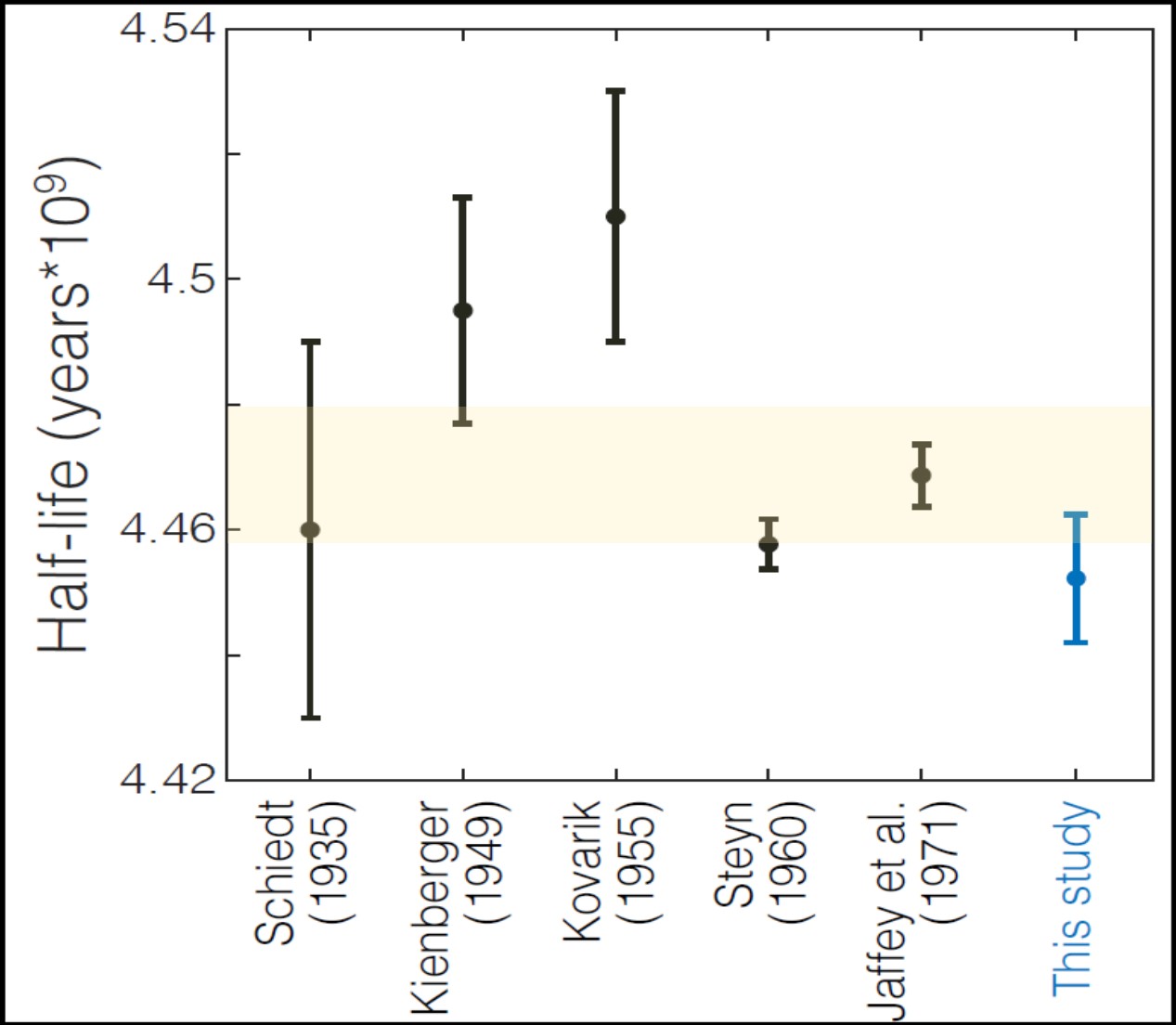
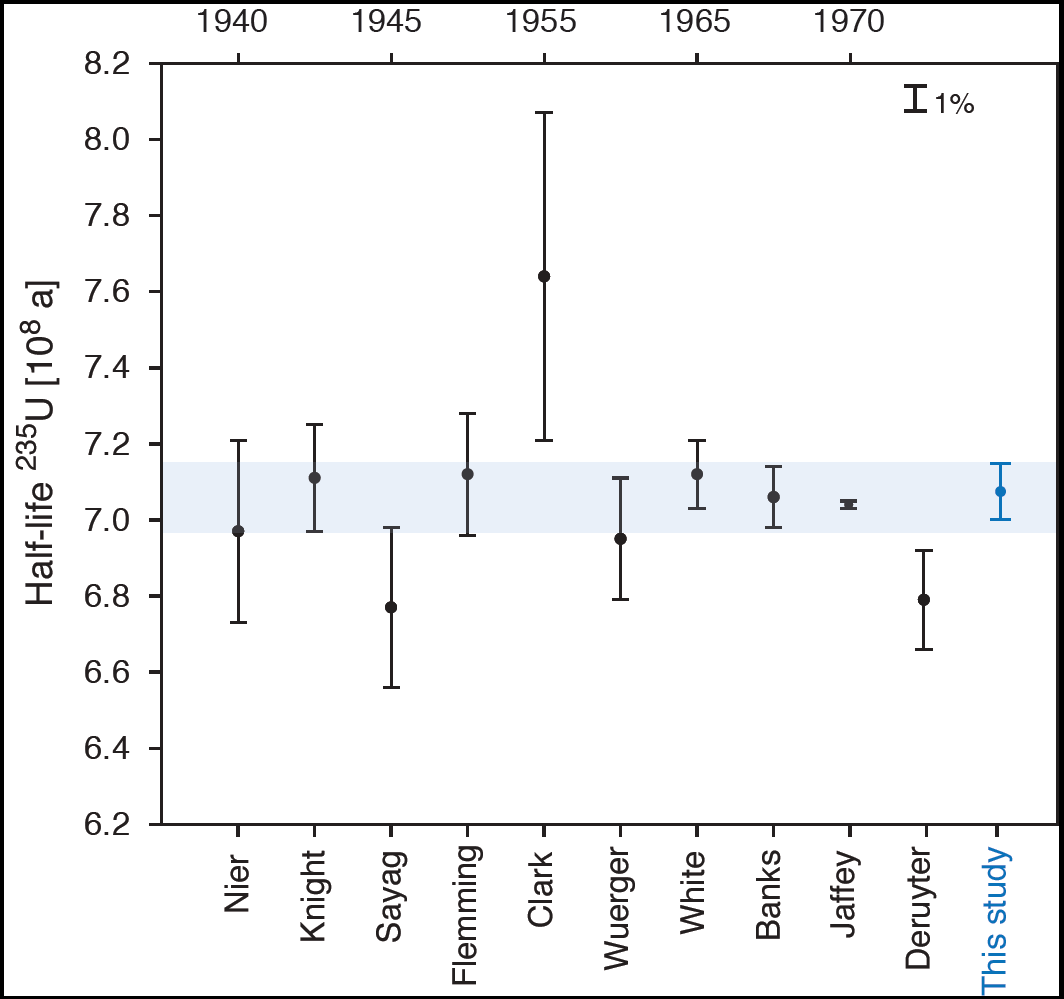
Problems were experienced with the chemical reagents during the second extraction in one of the middle aliquots (t = 87.6 ± 07 days); thus the result was discarded as an outlier. The average results from the other five aliquots yielded the decay constant 4.934 ± .011 E-18 inverse seconds, 0.37 ± 0.24 percent larger than that previously reported from alpha counting, with relative uncertainty of 0.23% 2-sigma. The uncertainty of the average was not greatly reduced from the uncertainties of the individual data points because of correlations, and the results from aliquots with ingrowth times less than 68 days were slightly discordant (smaller decay constants) with those from aliquots with ingrowth times greater than 108 days. The data do not reflect a trend that would be consistent with an inaccurate Th-234 decay constant. Possible reasons for the discordance are being considered, and the final error bar on the average value may be adjusted and increased to reflect the possibility of a systematic bias. Figure 2 shows our preliminary result, converted to a half-life of 4.452E9 ± 1.0E7 years, in context with previously reported and evaluated alpha-counting results (from Schoen et al. 2003). Of these, only our measurement and two others (Kienberger 1949 and Jaffey 1971) were direct measurements of enriched U-238, uncorrelated with natural uranium isotope ratios. Our results indicate slightly faster decay of U-238 than those obtained by alpha counting but overlap with the evaluated error envelope (shaded light yellow in Figure 2, from Villa et al. 2016) within two-sigma analytical uncertainty. These independent results underscore that small relative uncertainties and results from a single study should be used only with great caution, as systematic biases can be imagined in both types of experiments. For example, the alpha sources counted in Jaffey 1971 were thick (0.3–0.5 mg per square cm), while self-absorption affects in alpha sources are observed starting with thicknesses around 50 μg per square cm. It is possible that alpha particle scattering within the sources caused slight loss of alpha particles entering the detector at the defined solid angles, and modern modelling software such as GEANT4 could be used to determine if this was likely. Notably, our determined half-life for U-238, while slightly discordant with Jaffey 1971, is in agreement with the absolute activity measurement of Steyn and Strelow 1960 via liquid scintillation counting, a method unsusceptible to the scattering and self-absorption that may have affected the solid alpha sources of Jaffey 1971.
Figure 2. U-238 half-life determined in the present study (blue) and previously reported from alpha-counting experiments, with first author and year listed, adapted from (Schoen et al. 2003). Of these, only our measurement and two others (Kienberger 1949 and Jaffey 1971) were direct measurements of enriched U-238, uncorrelated with natural uranium isotope ratios. All error bars represent 2-sigma confidence intervals, and the evaluated error envelope (from Villa et al. 2016) is shaded orange.
Th-234 Half-life Measurements
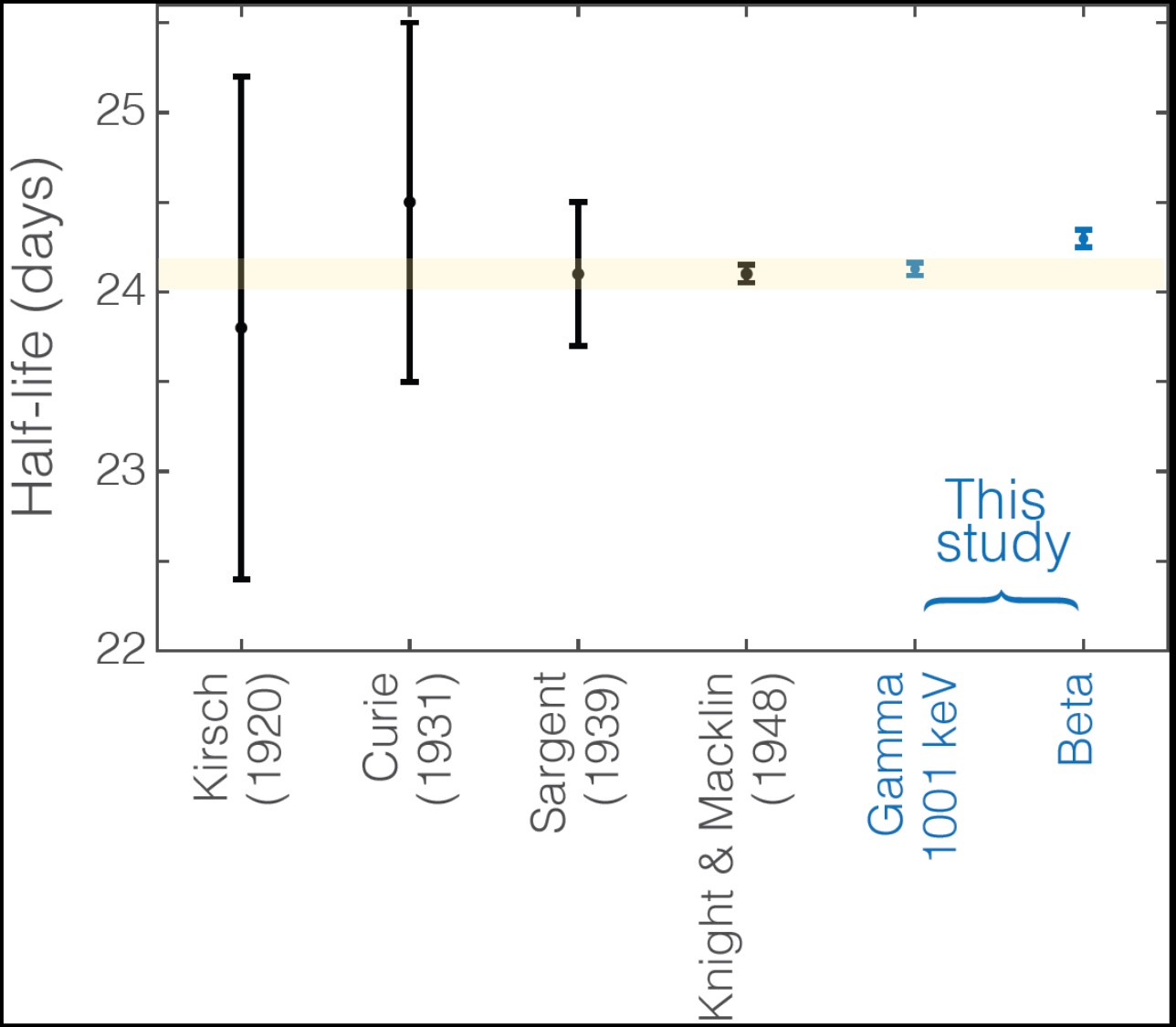
We have directly measured the decay curve of Th-234 sources that were extracted from depleted uranium to confirm the evaluated half-life of Th-234 (24.10 ± 0.06 days, Luca 2010), and potentially reduce its uncertainty. Sources were extracted by lanthanum fluoride co-precipitation with multiple washes and purified by anion exchange in 9 M HCl, followed by anion exchange in 8 M nitric acid. Thorium-oxide counting sources were prepared by molecular deposition onto aluminum disks (Parker et al. 1964), or, when sets of sources with known mass ratios were desired for checking pile-up effects, by drop-deposition from a weighed pycnometer onto stainless-steel planchettes. In the latter case, 10 ppm Zr carrier solution was used. The source surfaces were protected by affixing 3.6-micron film of Mylar. Decay curves were measured both by beta-counting in a windowless gas-proportional counter and gamma counting with HPGe detectors, fit to an exponential function with a constant background term. Residuals were plotted and evaluated, and the potential sources of error considered (Pommé 2015a). A total of ten counting sources were counted in a Protean IPC-650HP-02 windowless gas-proportional counter with P-10 gas (10% methane, 90% argon) over three measurement campaigns lasting 3–4 months each. The automated sample-change mechanism rotated samples, as well as a blank and uraninite, Tc-99, and Sr-90 check sources, and each source was counted two hours. The last of these measurements continues, but the instrument lacks the required precision, as scatter from check sources exceeds that expected from counting statistics and pulse-pileup appears to be an issue. A higher-activity source was counted in a close position with a large coaxial HPGe detector, using 10 cps pulser input for timing and electronic stability checks (Ortec DSPEC electronics), and an Am-241 source as a check on overall detector stability, including the crystal. A data-acquisition loop was set up in the Maestro software to save the spectrum and restart the count every two hours, and the peaks were fit to a Gaussian function in Peakeasy. The preliminary results (Figure 3) from the Pa-234m 1001-keV gamma peak (in secular equilibrium with Th-234) agree well with the most recent measurement reported by (Knight and Macklin 1948) and the currently recommended value (Luca 2010). Other peaks from the first campaign are being evaluated, and a new source is now being counted from a greater distance with a broad-energy Ge detector.
Figure 3. Th-234 half-life determined in the present study (blue) and previously reported counting experiments, with first author and year listed, adapted from (Luca et al. 2010). All error bars represent 2-sigma confidence intervals, and the evaluated error envelope (from Luca et al. 2010) is shaded orange.
U-234 and U-235 Counting-Source Preparation
The decay constants of U-234 and U-235 were evaluated by measuring the absolute activities of sources with precisely quantified amounts of isotopically enriched U (99.99% U-234 and 99.94% U-235). This requires sources that are precisely quantified, well-defined, homogenous, and stable—a significant challenge. The samples were chemically purified with anion-exchange procedures in HCl and nitric acid, dissolved in 4 M nitric acid with trace HF, and assayed by ID-MC-ICP-MS using IRMM-3636 spike, or with a different U-233 spike that was calibrated against CRM 112A and NBS 960 U metal standards. Some aliquots of U-234 were weighed for preparation of sources by molecular deposition onto a polished Al disk (Parker et al. 1964; Jaffey et al. 1971), which offers a defined area and usually better homogeneity, but has the disadvantage that the yield must be accurately quantified. A plating cell modeled after that of Jaffey et al. 1971 was machined out of polyethyl ether ketone (PEEK), as previous attempts with cells made from PFA or polytetrafluoroethylene (PTFE) Teflon resulted in unwanted deposition of Teflon onto the source. The residual solution was recovered, along with solution from several boiling steps for U-ID assay, and at least seven rounds of electrodeposition blanks were plated to recover and quantify trace uranium. The plating yields were approximately 90%, and a secondary method was needed to avoid uncertainties associated with quantifying the molecular-plating yield.
Thus, additional U-234 and U-235 sources were prepared by drop-depositing standardized solution from a weighed pycnometer to polished stainless-steel disk, pretreated with a wetting and seeding agent, and drying at 55 degrees C (Van Ammel et al. 2011). With this method, the source mass is more precisely quantified, but it is difficult to obtain homogenous sources with a well-defined area, and the long-term stability of such sources has not been documented. Substrates were characterized by optical and scanning electron microscopy, and digital autoradiography was used to characterize the distribution of activity on the counting sources.
U-235 Decay constant measurement
A challenge to measuring absolute activities with high precision is determining absolute detection efficiency without relying on standards, which are typically only accurate within a few percent uncertainty. An alpha-gamma coincidence counting system was developed and assembled, which allows mathematical elimination of the absolute efficiency term. The absolute activity of the source can be calculated by multiplying the individual alpha and gamma count rates and dividing by the coincidence count rate, all dead-time and background corrected (Schoen et al. 2004). U-235 is well suited for this method, because of the relatively high intensity of gamma emissions associated with its alpha decay.
In our current system, sources are firmly positioned in an adjustable-height sample holder inside a vacuum chamber with a down-looking, ion-implanted surface-barrier Si detector (Ortec ULTRA-AS, 1200 square mm area, 100-micron depletion thickness) facing the U side and an n-type HPGe detector (Ortec, 60% relative efficiency) beneath. Sources were counted for several weeks each in list-mode, using a FAST ComTec MPA-3 data-acquisition system with Model 7072 fixed conversion-time ADCs. The list data were processed using custom C++ codes in the Root data analysis platform and used the 143 keV gamma peak for U-235.
Preliminary results yield a half-life of 7.074E8 ± 7.3E6 years, which, as shown in Figure 4, is consistent with previously reported results, but not nearly precise enough for independent confirmation of Jaffey 1971. Two issues must be solved for improved precision of this measurement. The first is that the isotopic purity of the material is limited at 99.94%. The 234/235 isotopic ratio is 1.768E-4 ± 4E-6, which means approximately one-third of the total alpha activity comes from U-234 decay, and the Si detector lacks sufficient energy resolution to accurately resolve alpha particles from the two isotopes. The contribution of U-234 alpha activity had to be calculated from ICP-MS data and the evaluated-literature decay constant of U-234, then subtracted from the total, limiting the accuracy and precision of the U-235 decay constant calculated. A sample of U-235 containing much less 234 is needed, but if that were unobtainable, the activity ratio could be measured directly using a magnetic microcalorimeter detector, which has high enough energy resolution to distinguish all alpha particles. The second improvement would be to use an alpha detector with a 4-pi solid angle of detection, so that the net effect of any angular correlations among alpha and gamma particles would become zero (Campion 1958). This would allow more of the gamma lines to be used and increase coincidence counting statistics and could be achieved by replacing the Si alpha detector with a liquid scintillation counter (LSC), where the aqueous source aliquot is placed inside the detector, a chamber filled with LSC cocktail.
Figure 4. U-235 half-life determined in the present study (blue) and previously reported from alpha-counting experiments, with first author listed and traversing the X-axis in order of publication date. Adapted from (Schoen et al. 2003). Of these measurements, only ours, Knight, Flemming, White, and Jaffey, are direct measurements of enriched U-235, uncorrelated with U-238. All error bars represent 2-sigma confidence intervals, and the evaluated error envelope (from Villa et al. 2016) is shaded blue.
U-234 decay constant and gamma branching-ratio measurements
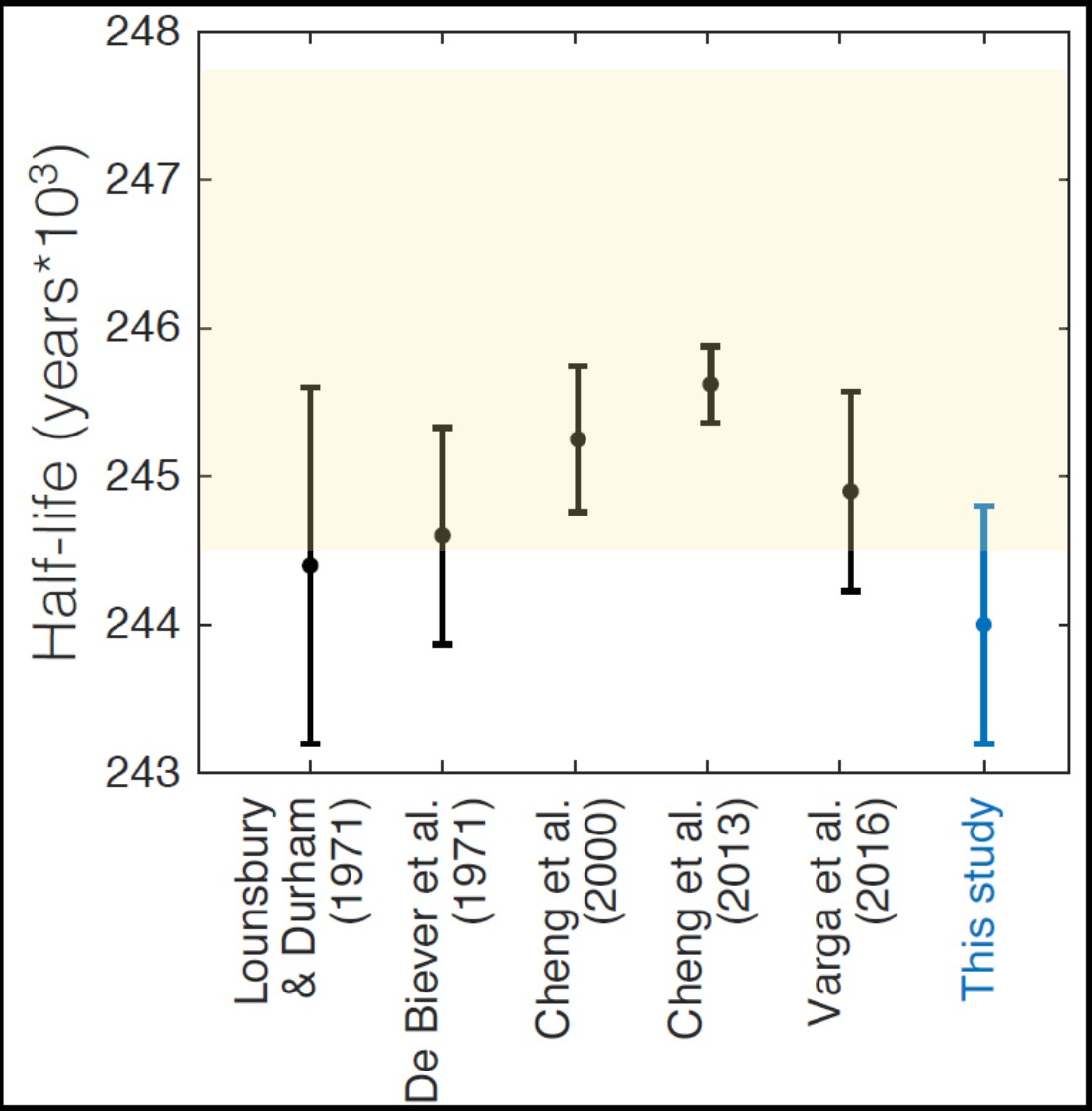
Alpha-gamma coincidence measurements were made on a source, but this method was found to be inappropriate for the absolute activity, due to the low branching intensities of the gamma emissions associated with U-234 decay. For absolute activity measurements of U-234, we used a low-geometry alpha counter, which rejects scattered alpha particles (or those emitted at low angles relative to the plane of the source) from the detector, via collimation with a precisely known aperture. The detection efficiency is reduced to first geometric principles and depends on precise measurement of the source radius, the collimator radius, and the distance between the two (Pommé 2015b). We originally intended to use a legacy counter for these measurements, but found it contaminated and unsuitable, so we designed and built a new system. The collimator radius was measured with NIST-calibrated gauge pins and the distance with NIST-calibrated gauge blocks, and the source radius is a precision-limiting factor. Preliminary results are plotted in Figure 5, along with previously reported results and the recently evaluated error envelope (Villa et al. 2016) that considered the results from geological samples (Cheng et al. 2000; 2013), along with observed variations in natural isotope ratios. Our preliminary results are discordant with those determined from geological samples but agree within uncertainty with the most recent alpha-counting results. The precision and accuracy of our results are expected to improve as more sources are counted and adjustments are made in the data analysis to better account for source heterogeneity by incorporating autoradiography data (Sibbens et al. 2003; Pommé 2015b).
Figure 5. U-234 half-life determined in the present study (blue) and previously reported counting experiments, with first author and year listed. All error bars represent 2-sigma confidence intervals, and the evaluated error envelope (Villa et al. 2016) is shaded orange.
The U-234 sources were counted with two different planar HPGe detectors to determine the gamma branching ratio of the 120.9 keV gamma emission, relative to the total alpha activity measured by low-geometry counting. Sources were measured at distances of 5 and 10 cm, and the detection efficiency for each gamma line was determined experimentally by preparation and measurement of two gamma standards to mimic the geometry of each U-234 source. These were prepared by deposition of a mass of depleted uranium, matching that of U-234 in the actual source, onto the same Al or stainless-steel backing, then drop deposition from a weighed pycnometer of a mixed-gamma reference solution from Eckert and Ziegler Isotope Products, certified by German Calibration Service (DAkkS). The mixed-gamma solution contained Co-57, which was used to calibrate the detectors at 122 keV, while the efficiency at 53.2 keV came from interpolation between 46 keV (Pb-210) and 59.7 keV (Am-241) calibration points. These measurements are still in progress, but preliminary results suggest branching ratios of 0.1222 ± 0.0054 percent and 0.03529 ± 0.0012 percent for the 53.2 and 120.9 KeV gamma emissions, respectively. These are in concord with previous evaluations (nucleide.org/DDEP; nndc.bnl.gov/ensdf) and provided greater than five-fold improvement in precision for the 120.9-keV gamma branching ratio.
Impact on Mission
The project achieved an important contribution in developing and expanding Livermore’s state-of-the art analytical measurement capabilities for nuclear forensics, radionuclide metrology, and standards development. Our mass-spectrometry team is already engaged with standard-reference-material development projects for nuclear forensics and safeguards applications, and several sponsors have expressed interest in funding related projects in the coming years. This project promoted meticulous consideration of all analytical methods and standards, further strengthening areas in which we excelled. It also brought new capabilities through the building of the low-geometry alpha counter and alpha-gamma coincidence counting system. The project promoted knowledge transfer from senior to early-career staff and contributed to the career development of three post-docs, including the PI, who was recently converted to staff. This research nurtured the Laboratory's collaborative relationship with the University of California, Berkeley, and the Berkeley Geochronology Center, which has historically brought quality talent to Livermore, and indirectly led to the hiring of an already indispensable staff member in the mass-spectrometry group.
Conclusion
The preliminary results presented here may be refined as final measurements and improved calculation methods are implemented. Their publication in the peer-reviewed literature will be valuable to the many users of these constants and, pending evaluation, these results may be incorporated into new recommendations. This is expected to lead to smaller recommended uncertainty on the Th-234 half-life and the U-234 gamma branching ratios. More work is needed to refine and verify the U-238 and U-235 decay constants. Our partners at the Berkeley Geochronology Center are in communication with the National Science Foundation regarding the possibility of additional funding. Incorporation of LSC detectors for increased detection efficiency, coupled with MMC detectors for superior resolution, may be a promising avenue for new alpha-counting experiments. The Livermore team will remain engaged with future efforts on uranium isotopes and will likely work on other radionuclide metrology projects in coming years.
References
Bibby, R., et al. 2010. "Nuclear Smuggling International Technical Working Group (ITWG) Round Robin #3." Lawrence Livermore National Laboratory. TR-434300.
Campion, P. J. 1959. “The Standardization of Radioisotopes by the Beta-Gamma Coincidence Method Using High Efficiency Detectors.” International Journal of Applied Radiation and Isotopes 4:232–248.
Cheng, H., et al. 2000. “The Half-Lives of Uranium-234 and Thorium-230.” Chemical Geology 169: 17–33.
Cheng, H., et al. 2013. “Improvements in 230Th dating, 230Th and 234 Half-Life Values, and U-Th Isotopic Measurements by Multi-Collector Inductively Coupled Plasma Mass Spectrometry.” Earth and Planetary Science Letters 371: 82–91.
De Bievre, P., et al. 1971. “The Half-Life of 234-U.” In Proc. Int. Conf. Chem. Nucl. Data, Measurement and Applications, Canterbury. Inst. Civil Engineers, edited by Hurrell, M.L., Ed 221–225. London.
Decay Data Evaluation Project, accessed October 2017, http://www.nucleide.org/DDEP_WG/DDEPdata.htm.
Harrison T. M., et al. 2015. “It’s About Time: Opportunities and Challenges for U.S. Geological Survey.” Institute of Geophysics and Planetary Physics Publication 6539, University of California, Los Angeles.
Jaffey A. H., et al. 1971. “Precision Measurement of Half-Lives and Specific Activities of 235U and 238U.” Physical Review C4 (5):1889–1906.
Lounsbury, M., and Durham, R.W. 1971. “The Alpha Half-Life of 234U.” In Proc. Int. Conf. Chem. Nucl. Data, Measurement and Applications, Canterbury. Inst. Civil Engineers, edited by Hurrell, M.L., Ed 215–219. London.
Ludwig, K. R. 2003. “Mathematical-Statistical Treatment of Data and Errors for 230Th/U Geochronology.” Reviews in Mineralogy and Geochemistry 52(1): 631–656.
Metropolis N. and Ulam, S. 1949. “The Monte Carlo Method.” Journal of the American Statistical Association, 44:247, 335–341.
National Nuclear Data Center NuDat Nuclear Structure and Decay Database, accessed October 2017, http://www.nndc.bnl.gov/nudat2/.
Parker, W., et al. 1964. “Molecular Plating I, A Rapid and Quantitative Method for the Electrodeposition of Thorium and Uranium.” Nuclear Instruments & Methods 26: 55–60.
Pommé S. 2015a. “The Uncertainty of the Half-Life.” Metroligia 52: S51–65.
Pommé S. 2015b. “The Uncertainty of Counting at a Defined Solid Angle.” Metroligia 52: S73–85.
Renne, P. R., et al. 2010. “Joint Determination of 40K Decay Constants and 40Ar/40K for the Fish Canyon Sanidine Standard, and Improved Accuracy for 40Ar/39Ar Geochronology.” Geochimica et Cosmochimica Acta 74(18): 5349–5367.
Schoene, B., et al. 2006. “Reassessing the Uranium Decay Constants for Geochronology Using ID-TIMS U-Pb Data.” Geochimica et Cosmochimica Acta 70: 426–445.
Schoen, R., et al. 2003. “A Critical Review of Experimental Data for the Half-Lives of the Uranium Isotopes 238U and 235U.” Applied Radiation and Isotopes 60:263–273.
Sibbens, G., et al. 2003. “Tailoring Solid Angle Calculations to the Actual Radioactivity Distribution of Planar Sources.” Nuclear Instruments and Methods in Physical Research A 505: 277–281.
Steyn, J., and F.W.E. Strelow, 1960. “The Determination of the Half-Life of 238U, Absolute Counting of Alpha Particles in a 4-Pi-Liquid Scintillation Counter.” In Metrology of Radionuclides. IAEA STI/PUB/6, edited by A. Sanielevici,155–161. Vienna.
Van Ammel, R., et al. 2011. “Preparation of Drop-Deposited Quantitative Uranium Sources with Low Self-Absorption.” Nuclear Instruments and Methods in Physics Research Section A 652: 76–78.
Villa, I.M., et al. 2016. “IUPAC-IUGS Status Report on Half-Lives of 238U, 235U, and 234U.” Geochimica et Cosmochimica Acta 172: 387–392.
Publications and Presentations
Parsons-Moss, T. 2016. “Modern Measurements of Uranium Decay Constants to Support High-Precision Radiochronometry.” Annual Academic National Laboratory Collaboration Meeting, Los Alamos, NM, August 3. LLNL-PRES-698180.
Parsons-Moss, T., et al. 2015. “Modern Measurements of Uranium Decay Rates.” American Geophysical Union Fall Meeting, San Francisco, CA, December 18. LLNL-ABS-678613.
——— 2016. “Modern Measurements of Uranium Decay Rates.” 252nd American Chemical Society National Meeting and Exposition, Nuclear Division, August 22. LLNL-ABS-690945.
——— 2017. “New Measurements of Uranium Decay Constants.” 6th Asia-Pacific Symposium on Radiochemistry (APSORC), International Conference Center, Jeju Island, Korea, September 21. LLNL-ABS-725555.