Sarah Baker (14-ERD-010)
Abstract
In this project we explored enzyme-catalyzed methane conversion to methanol. Industrial biological approaches to methane conversion using whole organisms are predicted to be more energy-efficient than chemical approaches, but are limited by mass transfer of the gas phase reactants, methane, and oxygen, to the organisms. We demonstrated that three-dimensional printing of the enzyme particulate methane monooxygenase (pMMO) embedded in a polymer can improve the kinetics of methane-to-methanol conversion. This improvement was likely because of the ability to increase the surface area of the catalytic material using three-dimensional printing. We also demonstrated the first continuous use of particulate methane monooxygenase in a flow-through reactor. In order to understand its fundamental kinetic properties, we conducted an in-depth study of its kinetics using analytical tools developed in our laboratory. Finally, we developed a new copolymer system that allowed tuning of the biocatalytic material’s gas permeability.
Background and Research Objectives
Natural gas flaring results in the release of about 13 million tons of carbon dioxide annually. A technology to efficiently convert methane to other hydrocarbons is highly sought-after to convert “stranded” sources of methane and natural gas (sources that are small, temporary, or not close to a pipeline) to liquids for further processing. Such a technology would also provide an incentive to reduce methane emissions from landfills and wastewater treatment plants. Methane is a potent greenhouse gas, with about 70-fold higher global warming potential than carbon dioxide, and emissions must be reduced to achieve the climate goals of the Paris Agreement. The only known true catalysts (industrial or biological) to convert methane to methanol under ambient conditions with 100% selectivity are monooxygenase enzymes, which perform the conversion in methanotrophic bacteria. Therefore, industrial biological methane conversion is projected to have significantly lower energy and capital costs than chemical conversion. However, the current reactors for biological methane conversion have low throughput and suffer from mass-transfer limitations.
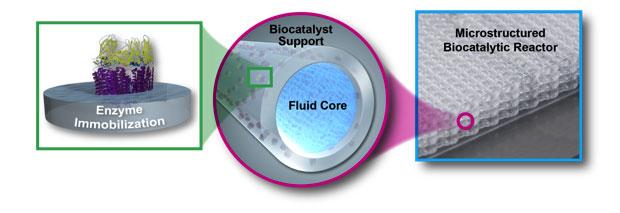
In this project we sought to explore (1) the properties and kinetics of isolated pMMO for converting methane to methanol, which enables a higher carbon conversion efficiency than whole cell conversions; (2) new polymeric materials; and (3) manufacturing methods to enable new reactor design to overcome throughput and mass-transfer limitations (an example reactor design is shown in Figure 1). We have achieved all of our goals for the project. In addition, we have filed one patent and published one high-impact paper describing results from objective 3. We plan to submit two additional papers describing results from objectives 1 and 2.
Scientific Approach and Accomplishments
Objective 1. Kinetics of Particulate Methane Monooxygenase
Despite decades of research into determining the location and nature of the active site in pMMO, an in-depth study of the enzyme kinetics has not yet been published. However, in order to design materials and reactors around its methane oxidation capability, the enzyme kinetics must be understood. For example, a reactor might be designed to improve mass transfer, or to reduce product inhibition, if these kinetic and mechanistic parameters were known. Therefore, one of the core objectives of this project was to uncover the kinetics, and therefore the overall mechanism and rate-limiting steps involved in methane oxidation catalyzed by this remarkable enzyme.
We developed two new methods for measuring the kinetics of pMMO. First was a new stopped-flow spectrophotometric assay that measures the rate of reducing agent (NADH) consumption as a proxy for methanol production. Second was measurement of the rate as a drop in pressure in the headspace over a reaction vial containing pMMO and the gas phase reactants over time. Importantly, we have verified that the kinetic parameters determined from both of these methods were similar. We have tested several potential mechanisms against the data, and found a mechanism that corresponded well. This mechanism suggests that activation of oxygen is likely the slow step in methane oxidation, and that this oxidation step is irreversible. Furthermore, the magnitude of the kinetic constants show where the concentrations of reactants become limiting, which will be informative for reactor design. Finally, the data indicate that product inhibition is an important factor influencing pMMO activity. These findings will have implications for design of both methane oxidation catalysts and bioreactors.
Objective 2. Polymer Design: Tuning the Properties of the Biocatalytic Material
The nearly limitless range of properties accessible using synthetic polymers and hydrogels can be coupled with the extraordinary catalytic properties of enzymes to create biocatalytic materials with enhanced function. We sought to create such a biocatalytic material by embedding cross-linking pMMO in polymers with tunable properties. Because methane conversion requires gas phase reactants, we focused on tuning the gas permeability. After finding that pMMO retained up to 100% physiological activity in a polymer composed of polyethylene glycol, we focused on synthesizing polymers that contained amphiphilic mixtures of polyethylene glycol, to retain enzyme activity, and polydimethyl siloxane, to provide gas permeability.
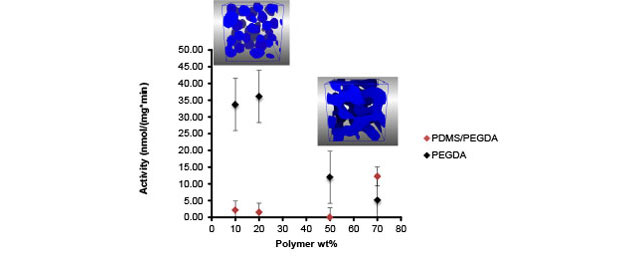
A critical challenge in the synthesis of polydimethyl siloxane–polyethylene glycol block copolymers suitable for cross-linking enzymes is that the material must be water-soluble to mix it with aqueous enzymes, and must be curable under mild conditions to preserve the enzyme activity. In fact, to our knowledge there are no reports of cross-linking enzymes in polyethylene glycol–polydimethyl siloxane block copolymers in the literature. We discovered that certain random brush copolymers satisfied both of these criteria. The polydimethyl siloxane–polyethylene glycol copolymer materials had tenfold higher gas permeability than polyethylene glycol alone. Our mesoscale model of one of the polymer variants, corroborated by x-ray scattering data, indicates that the polymer forms a network of polydimethyl siloxane and polyethylene glycol domains at high volume percent of polymer (>50%) in water (Figure 2, inset). We successfully incorporated pMMO into this polymer and measured the activity as a function of polymer volume percent. Our major finding was that the activity, while lower than that of pMMO in polyethylene glycol at lower polymer concentrations, increased as the polymer concentration increased, the opposite of the trend seen in polyethylene glycol (Figure 2). Combined, these results indicate that tuning the network structure of polymers at the nanoscale may allow tuning of the biological activity of enzymes embedded in them.
Objective 3. Tuning the Polymer/Reactor Architecture Using Three-Dimensional Printing
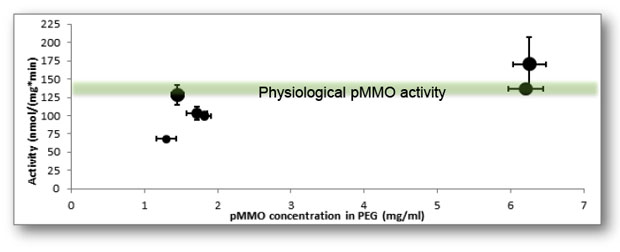
In an effort to develop a biocatalytic material that can be molded into controlled, predetermined structures with tunable surface areas, we created a polymeric material based on polyethylene glycol, which could be embedded with pMMO and used as a feedstock for three-dimensional printing (Figure 3). We discovered that at low polymer concentrations, the pMMO–polyethylene glycol material had the same catalytic activity as pMMO in the cell. We explored printing this material into geometries with varying surface area, and found that the pMMO activity increased as the surface area of the material increased (Figure 4). Furthermore, we found that this printed material allowed high loadings of pMMO, enabling high volumetric productivity. Our results with printed pMMO show that the strategy of printing catalysts into reactor geometries that are designed for maximally efficient use of reactants, energy, and reactor volume has promise both for methane conversion and other biocatalytic processes.
The synthesis of the catalytic polymer material allowed pMMO to be used in a continuous reactor for the first time rather than in a batch process. The ability to print the pMMO membrane in three dimensions allowed a comparison of different membrane thicknesses suspended at the gas–liquid interface. We found that thinner printed membranes produced more methanol over time, consistent with the previous results that suggested the pMMO is mass-transfer limited, and increasing the surface area is a promising approach to utilizing pMMO most effectively.
Impact on Mission
This project is directly relevant to the Laboratory’s focus on materials for energy. Specifically, it has increased Laboratory expertise in the strategic focus areas of climate and energy security, and engineered materials. In addition, the project demonstrated a new application for the Laboratory’s capability in engineered and manufactured materials for advanced manufactured reactors. We will continue to explore this area for other energy-relevant applications. We have hired and supported two new postdoctoral staff members on this project.
Conclusion
We have demonstrated that polymer synthesis and advanced manufacturing enable the properties of a biocatalytic material to be tuned to the application. Specifically, we showed that pMMO can be embedded in a polymer while retaining its native activity, and that printing this biocatalytic polymer into high-surface-area structures with high enzyme loading improves this activity. The kinetic model that we developed for pMMO will be instructive both for fundamental understanding of the enzyme, and for the design of bioreactors that use pMMO. The advanced manufactured bioreactor concept can be relevant to many applications, including microbial electrosynthesis and syngas fermentation. Co-development of the materials, the manufacturing, and the necessary biocatalysts are needed to realize this technology commercially and to expand the use of enzymes for energy applications.
Publications and Presentations
- Blanchette, C., et al., “Printable enzyme-embedded materials for methane to methanol conversion.” Nat. Comm. 7, 11900 (2016). LLNL-JRNL-678269. http://dx.doi.org/10.1038/ncomms11900
- Baker, S. E., et al., Biocatalytic polymer materials for partial oxidation of methane to methanol. 249th American Chemical Society National Mtg. and Expo., Denver, CO, Mar. 22–26, 2015. LLNL-PRES-668366.
- Baker, S. E., et al., Printable, biocatalytic polymer materials for partial oxidation of methane to methanol. 2015 MRS Fall Mtg. and Exhibit, Boston, MA, Nov. 29–Dec. 4, 2015. LLNL-ABS-676428.