Philip Pagoria (15-ERD-044)
Abstract
With few investigators in the U.S. developing new energetic compounds, we plan to investigate the use of new melt-castable explosives as possible detonator and booster candidate materials relevant to high-explosives applications. With this project, we assessed the viability of these melt-castable materials by determining their volume change, crystallization properties, and the use of additives and eutectics (mixture of substances having a minimum melting point) to improve material performance. An optimized method for casting these materials was also explored. Because these new compounds have relatively low melting points (80–109oC), and are stable in the melt, they present some interesting possibilities with respect to material processing. The possibility exists for these materials to be cast into various forms, formed into precise shapes using lithographic techniques, or possibly used in additive manufacturing techniques to produce specialized three-dimensional shapes. We investigated these materials and their mixtures and performed an initial evaluation as to their suitability as melt-castable materials.
Background
New booster and detonator materials are needed as part of the Stockpile Surveillance Program and for future stockpile considerations. Currently, Lawrence Livermore is one of only two national laboratories performing basic research in the development of new energetic compounds in the U.S. Our goal is to synthesize new energetic materials and perform an assessment of their viability as ingredients for tailored materials and use in additive manufacturing applications. If successful, these materials could be of great importance to weapon designers, replacing some of the current materials with compounds that have higher-performance and enhanced properties. These materials may be used to form three-dimensional structures by melting and casting the material (melt-castable material) into a desired shape, with minimal waste and in a reproducible manner. In addition, they may be incorporated into additive manufacturing technology in which they can be delivered, using heated devices, into three-dimensional monoliths and substrates. One of the interesting aspects of these materials is that the differing melting points may allow layering or adding these materials into a mold sequentially, possibly allowing tailoring of performance and sensitivity.
Recently, the Energetic Materials Synthesis Group at LLNL synthesized a series of melt-castable compounds of similar structure to 2,4,6-trinitrotoluene (TNT), with superior performance and insensitivity compared to the industry standard. The properties can be tailored through the formation of mixtures and eutectics of these compounds, which are a stable mixture of materials that, when combined, act as a single-component material. These materials, because of their low melting point, may also be delivered using additive manufacturing techniques to form custom three-dimensional structures with tailored performance, along with improved control of properties and reproducibility.
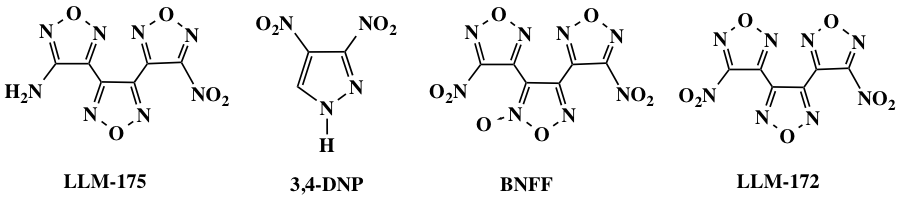
The four candidate compounds we identified were 3-(4-amino-1,2,5-oxadiazol-3-yl)-4-(4-nitro-1,2,5-oxadiazol-3-yl)-1,2,5-oxadiazole (LLM-175); 3,4-dinitropyrazole (3,4-DNP); 3,4-bis(4-nitro-1,2,5-oxadiazol-3-yl)-1,2,5-oxadiazole-2-oxide (BNFF); and 3,4-bis(4-nitro-1,2,5-oxadiazol-3-yl)-1,2,5-oxadiazole (LLM-172), as shown in Figure 1.1–4 Our goal was to assess the viability of these melt-castable materials by determining their volume change, crystallization properties, and the use of additives and eutectics to improve material performance. An optimized method for casting these materials was also explored. The synthetic methods to produce these compounds were demonstrated, and although they continue to undergo optimization, especially in the purification stage, we determined that large quantities of these compounds may be synthesized in a safe and timely manner using known procedures.
The compounds were chosen for their promising performance and safety properties. BNFF is a promising detonator candidate with excellent thermal stability and good safety properties. With a density of 1.875 g/cm3, it is a high-performance energetic compound with a melting point of 109oC. It has a failure diameter of about 100 µm (smaller than CL-20),5 yet has sensitivity similar to HMX (octogen, a powerful and relatively insensitive nitroamine high explosive). BNFF has been of interest as a melt-castable material in both China and Russia: Zhou6 and Stepanov7,8 investigated the properties of BNFF along with the eutectic of BNFF with TNT.
LLM-172 has a density of 1.836 g/cm3 and a melting point of 84oC, and 3,4-DNP has a density of 1.791 g/cm3 and a melting point of 85oC. Both of these compounds have good thermal stability and power approaching HMX, and may have applications as booster or main-charge energetic materials.
In addition to being melt-castable materials, BNFF, LLM-172, and 3,4-DNP also have the attractive property of being stable in the gas phase and so may be sublimed and deposited onto surfaces in a controlled fashion. One can envision using lithography technology, incorporating masks to produce lines or other precise three-dimensional patterns.
LLM-175 is a promising insensitive energetic compound with a melting point of 100oC. It has a density of 1.782 g/cm3, power similar to RDX (an explosive nitroamine widely used in military and industrial applications), and exceeds normal standards on the drop hammer test4 (a steel weight free falling from a height onto a steel striker that transmits the impact to an explosive test sample). It was envisioned as a possible material that could be melt-casted into booster configurations to essentially full density. LLM-175 may also be sublimed and deposited onto surfaces using lithography technology in a manner similar to BNFF, LLM-172, and 3,4-DNP.
Scientific Approach and Accomplishments
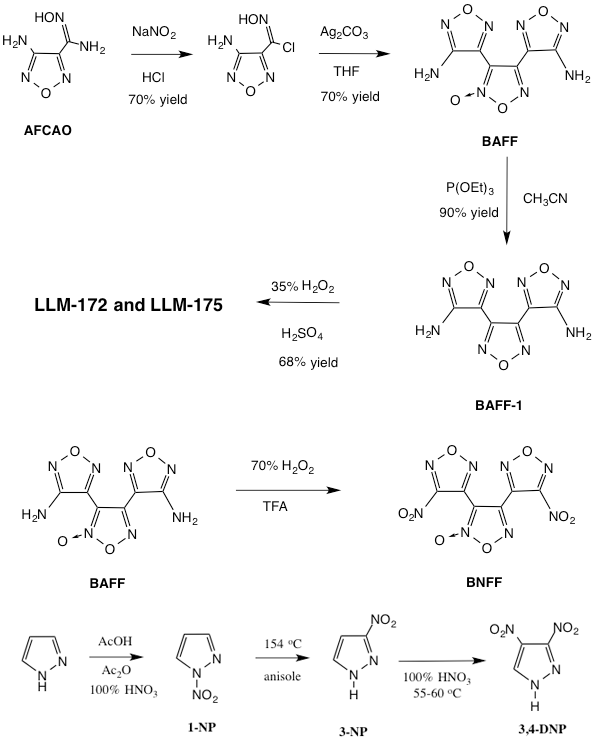
The first part of this research project involved the synthesis and purification of significant quantities of the four target molecules. The synthesis of LLM-172, BNFF, and LLM-175 all use the same intermediate compounds: 4-aminofurazan-3-carboxamidoxime (AFCAO) and 3,4-bis(4-amino-1,2,5-oxadiazol-3-yl)-1,2,5-oxadiazole-2-oxide (BAFF). Large quantities of AFCAO and BAFF were synthesized using procedures developed earlier in our laboratory. The AFCAO was synthesized at the 300-g scale per batch and the BAFF was synthesized at the 100-g scale per batch. Approximately 300 g of BAFF were synthesized to provide enough material for subsequent conversions. The synthetic conversions of these intermediates to our target compounds are shown pictorially in Figure 2.
The synthesis of energetic compounds such as BNFF, LLM-172, LLM-175, and 3,4-DNP must follow a safety protocol in which the syntheses are scaled-up in a stepwise fashion (1, 10, 50, and above 50 g). The procedures and high-explosives safety of the synthetic steps are examined by a set of peer reviewers at each scale-up phase, and safety data for each phase are collected. Approximately 60 g of LLM-172, 45 g of LLM-175, 25 g of BNFF, and 60 g of 3,4-DNP were synthesized (see Figure 2). A considerable amount of effort went into the purification of each compound to ensure that we were using very pure compounds for the formation of our eutectic and solid mixtures. We sublimed BNFF, LLM-172, and LLM-175 at reduced pressure at bath temperatures just above the melting point of each compound, yielding rather pure compounds that were subsequently recrystallized to yield a crystalline product. BNFF and LLM-172 were recrystallized from methyl-tert-butyl ether (MTBE) while LLM-175 was recrystallized from chloroform.
The synthesis of 3,4-DNP involves the nitration of 3-nitropyrazole with 100% nitric acid at 55 to 60oC, followed by removal of the nitric acid under vacuum to yield 3,4-DNP as a white solid. Recrystallization from trifluoroacetic acid yields 3,4-DNP as white microcrystals, which, after drying under vacuum, yield a pure compound that was used for eutectic and solid mixture formation.
The second part of this project was an assessment of these compounds for their effectiveness as melt-castable materials while also investigating interesting eutectics and solid mixtures of these materials (and possibly mixtures with other energetic compounds). To estimate the component mixture percentages that would produce eutectic mixtures of various components, we used the method of Chapman and Fronabarger.9 They reported a simple equation for the prediction of eutectic mixtures with a good correlation (r2 = 0.838):
ln[xsolute/x solvent] = −4077.04(DT-1) − 0.979
(where solvent is the lower-melting component). For mixtures of DNTF, LLM-172, and LLM-175, the molar percentages of each component using the above method are:
- DNTF/LLM-172: 31/69%
- LLM-175/LLM-172: 37/63%
- DNTF/LLM-175: 26/74%
- LLM-172/3,4-DNP: 48/52%
These values were used as a starting point for studying the various eutectic and solid mixtures. The Cheetah thermochemical code was used to predict performance for the candidate eutectics and solid mixtures, and the results helped guide selection of the solid mixtures and eutectics.
We used an Opti-Melt melting point apparatus to measure the melting points of a series of mixtures. The system allows recording and observation of the melting characteristics of the mixtures. The components of the mixtures were weighed and placed into a stainless-steel vial containing a stainless-steel ball bearing. The vial was placed into a “Wig-L-Bug” and allowed to shake for 30 s to thoroughly mix and pulverize the two components into a fine powder. This intimate mixture was then used for melting point determination. In many cases the samples were re-melted to confirm they melt at the same temperature after solidification. The solid mixtures that gave the sharpest melting point and seemed to be close to the eutectic mixtures were:
- DNTF/LLM-172 (mol%): 28.6/71.4 molar ratio that gave a relatively sharp melting point at 82 to 84oC. It was observed that BNFF seemed somewhat soluble in LLM-172 in the melt phase, which made the determination of the melt somewhat problematic
- LLM-172/LLM-175 (mol%): The 60.1/39.9 mixture gave a smooth melting point at 69 to 71oC
- DNTF/LLM-175 (mol%): The 22/78 mixture gave a smooth melting point at 82oC
- LLM-172/3,4-DNP (wt%): The 48/52 mixture gave a smooth melting point at 73oC
After we identified these mixtures of initial interest, we formulated the mixtures at the 2-g scale and submitted the formulations for small-scale safety tests. The formulations were made by dissolving the appropriate amount of each component in acetone and then removing the solvent under vacuum and heat, yielding an intimate mixture of the two components. We dried the formulation under vacuum at 30oC to ensure all the solvent was removed prior to submitting the sample for small-scale safety tests. These tests measure the response of the formulation to various stimuli (impact, friction, spark, and heat) to obtain an understanding of the relative sensitivity of the formulation to known explosives (see Table 1). The small-scale safety test results are also necessary before the formulation can be scaled-up further to 10 and 50 g.
Table 1. Small-scale safety tests results for individual energetic components and mixtures.
| Material | Drop hammer (cm) | Friction (kgs) | Spark | Melting point (oC) | Density (g/cm3) |
| 3,4-DNP | 60 | 1/10 @ 28.8 | No | 85 | 1.791 |
| LLM-172 | 43 | 1/10 @ 19.2 | No | 84 | 1.836 |
| LLM-175 | >177 | 1/10 @ 24 | No | 100 | 1.782 |
| BNFF | 31 | 2/4 @ 14.4 | No | 109 | 1.875 |
| LLM-172/ BNFF | 41 | 1/10 @ 16.0 | No | 84 | 1.852 |
| LLM-175/ BNFF | 72 | 1/10 @ 25.2 | Yes 1 go @ 0.25J | 81 | 1.784 |
| LLM-172/ 3,4-DNP | 137 | 1/10 @ 18.0 | No | 73 | 1.839 |
The solid mixtures of the various components had good small safety-data results. The mixture that stood out the most was LLM-172/3,4-DNP. There seemed to be a synergistic interaction between the two components, resulting in higher than anticipated density and significantly higher drop hammer values than the individual components. This mixture will be investigated more thoroughly in the future. The only negative result from the mixtures was from LLM-175/BNFF, which was found to be spark sensitive, even though the individual components did not show any spark sensitivity.
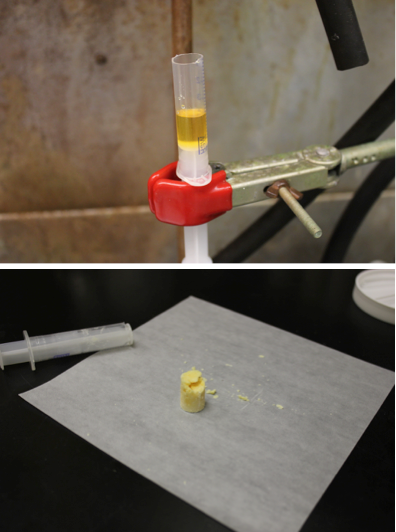
We also investigated whether these solid mixtures could form monoliths and estimated the amount of shrinkage that occurs with these mixtures upon going from liquid to solid phase. Each of the mixtures formed nice monoliths when the mixtures were melted into a plastic syringe, cooled, and the resulting solids extracted by cutting away the plastic syringe (see Figure 3). The samples crumbled slightly because of the force needed to cut the plastic, but generally held their shape well. In the future, the material would be poured into a mold that is sprayed with mold release and carefully extracted from the mold. The weakest monoliths were formed from pure LLM-172. The strongest monoliths seemed to be formed by 3,4-DNP.
Figure 3. LLM-175/LLM-172 mixture (3.5 g) in the melt phase (top) and after solidification when it has formed a monolithic piece (bottom).
Impact on Mission
Our research is aligned with the Laboratory's strategic mission focus in stockpile stewardship science and core competency in advanced materials and manufacturing, where new methods are needed to produce tailored materials with improved mechanical properties, performance, and safety. New booster and detonator materials are needed by weapons designers to meet the requirements for improving the safety and performance of munitions, along with the miniaturization of weapon systems. The use of melt-castable materials will improve reproducibility of parts and thus reduce future stockpile surveillance requirements.
Conclusion
We demonstrated the synthesis and purification of four candidate melt-castable explosives with attractive performance and safety characteristics. These materials could be of great importance to both DOE and Department of Defense weapon designers, replacing some of the current materials with higher-performance compounds with improved properties. These materials may find applications in the Laboratory's additive manufacturing core competency by providing an energetic material that may be melted into forms or other substrates. The results from this project are also of interest to sponsors in the Department of Defense and Defense Threat Reduction Agency, which share an interest in the development of new detonator and booster materials. These materials may also have application in the Department of Defense as replacements for the industry standards in melt-castable materials, TNT, and the high-performance insensitive high-explosive composite mixture IMX-101. The availability of new melt-castable materials with increased performance relative to current industry standards will provide an additional research avenue to further the state of the art of advanced high explosives.
References
- Wang, J., et al., Energetic materials: Insensitivity, ageing, monitoring: 37th Intl. Ann. Conf. ICT, p.171/1. Fraunhofer Institute for Chemical Technology, Karlsruhe, Germany (2007).
- Pagoria, P., et al., “3-(4-amino-1,2,5-oxadiazol-3-yl)-4-(4-nitro-1,2,5-oxadiazol-3-yl)-1,2,5-oxadiazole.” Molbank, M824, 1 (2014).
- Astrat'ev, A. A., D. V. Dashko, and A. I. Stepanov, “Synthesis, energetic and some chemical properties of new explosive—3,4-bis(4-nitrofurazan-3-yl)furazan (BNTF).” Proc. 16th Intl. Sem. New Trends in Research of Energetic Materials, p. 482. University of Pardubice, Pardubice, Czech Republic, P. S. Zeman, Ed. (2012).
- Pagoria, P. F., et al., " ‘Green’ energetic materials synthesis at LLNL.” Proc. 15th Intl. Sem. New Trends in Research of Energetic Materials, p. 54. University of Pardubice, Pardubice, Czech Republic, P. S. Zeman, Ed. (2012).
- Tappan, A., Sandia National Laboratories, Albuquerque, NM, private communication (2015).
- Zhou, W.-J., G. Zhang, and Z.-R. Liu, “Kinetics of non-isothermal crystallizations of BNFF, TNT and BNFF-TNT eutectic system crystallization in RDX.” Chin. J. Energetic Mater. 2008, 267 (2008).
- Stepanov, A. I., D. V. Dashko, and A. A. Astrat'ev, “3,4-bis(4'-nitrofurazan-3'-yl)furoxan: A melt cast powerful explosive and a valuable building block in 1,2,5-oxadiazole chemistry.” Cent. Eur. J. Energetic Mater. 9, 329 (2012).
- Stepanov, A. I., D. V. Dashko, and A. A. Astrat'ev, “Some chemical properties of 3,4-bis(3-nitrofurazan-4-yl)furoxan.” Proc. 15th. Intl. Sem. New Trends in Research of Energetic Materials, p. 301. University of Pardubice, Pardubice, Czech Republic, P. S. Zeman, Ed. (2012).
- Chapman, R. D., and J. W, Fronabarger, “A convenient correlation for prediction of binary eutectics involving organic explosives.” Propell. Explos. Pyrotech. 23(1), 50 (1998).