John D. Despotopulos | 17-LW-035
Overview
It is difficult to study the transactinides (elements 104 to 118) due to their low production rates and short half-lives. These elements are produced one atom at a time in particle accelerators; therefore, any chemical system developed must be extremely specific, automatable, and robust enough to conduct long-duration experiments. The goal of this project was to develop organic extractants for use in exploring the chemistry of Cn (element 112) and Fl (element 114). Previous studies have shown that crown ethers display the desired specificity for a Cn or Fl experiment but lack the kinetics (i.e., the speed of extraction) required to study an element that has a half-life of less than 30 seconds. Novel thiacrown-ether analogs of crown ethers were synthesized as part of this project. The sulfur donor atoms in the rings (as opposed to oxygen atoms as in traditional crown ethers) should interact more strongly with the homologs of Cn and Fl (Hg and Pb, respectively). As part of this project, we developed a chemical system for studying Cn based on a thiacrown ether with extremely fast kinetics and an affinity for Hg. We also developed a synthetic method for producing a thiacrown for use in exploring Fl.
Background and Research Objectives
The field of superheavy-element science has returned to the public eye with the recent announcement by the International Union of Pure and Applied Chemistry of the discovery of elements 113, 115, 117, and 118, officially filling the seventh row of the periodic table (Karol et al. 2016). Recent experiments in the field have focused on the discovery of element 119, the first element in the eighth period (Düllmann 2012), and the exploration of the chemical properties of the transactinides in order to establish their placement within the periodic table of elements. Due to the fact that transactinides have a large number of protons, the inner orbital electrons are accelerated to relativistic speeds, which can cause electron orbital contraction, thereby altering their chemical bonding characteristics and subsequent chemical behavior with respect to their lighter homologs (i.e., elements in the same chemical group) in the periodic table (Pitzer 1975). One of the most interesting cases of relativistic effects is predicted to occur with Fl. Group 14 of the periodic table begins with C, which exhibits a 4+ oxidation state, and ends with Pb, which exhibits a 2+ state. Relativistic calculations indicate that the 4+ state in Fl will be destabilized, leading to a more metallic characteristic for Fl that is similar to the properties of Pb (Seth et al. 1998). However, separately performed calculations indicate that Fl will display greater volatility and chemical inertness compared to typical Group 14 elements (Pitzer 1975), making it similar to a noble gas or perhaps Hg (Hoffman et al. 2006). Only gas-phase experiments have been performed with Fl, and the results so far are contradictory (Eichler et al. 2010, Yakushev et al. 2014). The results of one experiment performed by the Flerov Laboratory of Nuclear Reactions (FLNR) in Russia indicated that Fl had a relatively high volatility comparable to that of Hg (Eichler et al. 2010). Another experiment performed at Gesellschaft für Schwerionenforschung (GSI) in Germany indicated that it was less volatile than Hg (Yakushev et al. 2014), which is more in line with recent theoretical predictions (Pershina et al. 2009). These results indicate that the behavior of Fl is complex and that additional experiments are required to assess whether relativistic effects are modifying its chemical behavior from what would be expected of Group 14 homologs. The only studies of the behavior of Fl in the aqueous phase were those of crown ethers conducted at Lawrence Livermore National Laboratory and other studies of the production of radionuclides of the Fl homologs via radionuclide-generator systems.
Investigations of the chemistry of the transactinide elements present many challenges. The short half-lives and small cross sections at the nano- or picobarn levels result in low production rates, which means that transactinides can only be studied one atom at a time. Flerovium has a very short half-life (approximately three seconds) and a low production rate (approximately one atom per week); therefore, methods of study based on chemistry must be rapid and easily automatable to isolate single atoms produced during an experiment. Chemical properties can be determined by first studying the lighter homologs of Group 14 using a chemical extraction with very rapid kinetics. The chemical properties of Fl are then determined through comparison of its separation properties to that of its homolog elements. The GSI and FLNR laboratories are committed to performing future experiments designed to describe the aqueous-phase behavior of Fl. FLNR is building a new superheavy element factory, with a predicted production rate 100 times greater than current accelerators and that will support long-term chemical investigations of Fl. Our ongoing collaboration with both institutions, as well as contributions to heavy-element discoveries and expertise in rapid automated chemistry ideally position our team to perform the first aqueous-phase-based experiments on flerovium, which will impact models that define the limits of the periodic table of elements.
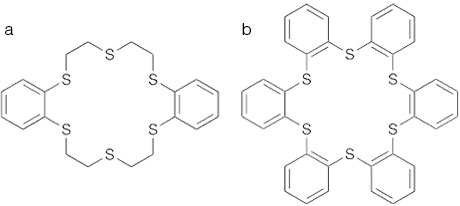
This project had two main objectives: (1) synthesizing novel thiacrown ethers (see figure) that were highly specific for the Fl homolog and pseudo-homologs (Pb and Hg, respectively); and (2) developing a chemical-separation method that can be automated and used to explore the chemical properties of Fl. Crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups. Thiacrown ethers are macrocycles (i.e., molecules containing twelve or more atoms with at least one large ring) where the oxygen atoms in a normal crown have been replaced with sulfur atoms, which have increased affinity for metals such as Hg and Pb. Unlike their oxygen counterparts, the charge density in thiacrown ethers is not directed toward the center of the cavity but instead is directed to the outside of the macrocycle (exodentate). We were able to synthesize thiacrown ethers that targeted, with extremely high specificity, the pseudo-homolog Hg and provided a platform for a study of element 112 (Cn). A lack of affinity for Pb may have been due to the exodentate nature of the S atoms on the original target molecule. To attempt to gain Pb extraction, we synthesized a series of different thiacrown ethers and S-containing organic chelators, which suppress chemical activity by forming ring-structured compounds containing a metal ion held by coordinate bonds. None of these thiacrowns showed promise for the extraction of Pb though each showed affinity for Hg. Finally, we designed a thiacrown ether that would force the S atoms to be endodentate (“b” in figure) and established a synthetic method of creating this molecule.
Target thiacrowns: ( a ) dibenzohexathia-18-crown-6 (with exodentate S atoms) and ( b ) hexabenzohexathia-18-crown-6 (with endodentate S atoms).
The extraction behavior and kinetics of the Fl and Cn homologs were explored along with the above-mentioned thiacrown ethers as part of this project. Isotopes of the Fl and Cn homologs were produced using previously established criteria (Despotopulos et al. 2015 and 2018). From this research, we were able to develop methods for exploring the chemistries of Cn and Fl.
Impact on Mission
Our research into the development of novel separation chemistry enhances the Laboratory’s core competencies in nuclear, chemical, and isotopic science and technology.
References
Despotopulos, J. D., et al. 2015. "Production and Isolation of Homologs of Flerovium and Element 115 at the Lawrence Livermore National Laboratory Center for Accelerator Mass Spectrometry." Journal of Radioanalytical and Nuclear Chemistry 308(2): 567–572. doi: 10.1007/s10967-015-4500-z.
——— . 2018. "Studies of the Homologs and Pseudo-Homologs of Flerovium with Crown Ether Based Extraction Chromatography Resins." Journal of Radioanalytical and Nuclear Chemistry. doi: 10.1007/s10967-018-6207-4.
Düllmann, C. E. 2012. "Search for Element 119." Presentation to the 11th Workshop on Recoil Separator for Superheavy Element Chemistry TASCA12.
Eichler, R., et al. 2010. "Indication for a Volatile Element 114." Radiochimica Acta 98(3): 133–139. doi: 10.1524/ract.2010.1705.
Hoffman, D. C., et al. 2006. "Transactinide Elements and Future Elements." The Chemistry of the Actinide and Transactinide Elements ." 1652–1752. doi: 10.1007/1-4020-3598-5_14.
Karol, P. J., et al. 2016. "Discovery of the Elements with Atomic Numbers Z = 113, 115 and 117 (IUPAC Technical Report)." Pure Applied Chemistry 88 (1-2): 139–153. doi: 10.1515/pac-2015-0502.
Pershina, V., et al. 2009. "Theoretical Predictions of Adsorption Behavior of Elements 112 and 114 and Their Homologs Hg and Pb." Journal of Chemical Physics 131(8): 084713. doi: 10.1063/1.3212449.
Pitzer, K. S. 1975. "Are Elements 112, 114, and 118 Relatively Inert Gases?" Journal of Chemical Physics 63(2): 1032–1033. doi: 10.1063/1.431398.
Seth, M., et al. 1998. "The Stability of the Oxidation State +4 in Group 14 Compounds from Carbon to Element 114." Angewandte Chemie International Edition 37(18): 2493–2496. doi: 10.1002/(SICI)1521-3773(19981002)37:18<2493::AID-ANIE2493>3.0.CO;2-F.
Yakushev, A., et al. 2014. "Superheavy Element Flerovium (Element 114) is a Volatile Metal." Inorganic Chemistry 53(3): 1624–1629. doi: 10.1021/ic4026766.
Publications and Presentations
Despotopulos, J. D., et al. 2017. "Studies of Flerovium Homologs with Thiacrown Ethers." Superheavy Elements 2017 (SHE 2017), Kazimierz Dolny, Poland, September 2017. LLNL-PRES-738124.
——— . 2018. "Studies of Flerovium Homologs with Thiacrown Ethers." American Chemical Society 255 th National Meeting, New Orleans, LA, March 2018. LLNL-PRES-747949.
Kmak, K. N., et al. 2017. "Separation of Pb, Bi and Po by Cation Exchange Resin." Journal of Radioanalytical Nuclear Chemistry 314(2): 985–989. LLNL-JRNL-731220.