Monica Moya (14-ERD-005)
Abstract
The development of engineered tissue that represents human physiology will help increase the quality and predictability of therapies that move through the Food and Drug Administration approval pipeline and into clinical use. In vitro (outside the body) tissue models to date feature engineered organs that survive for only a few weeks and grow, at most, to a few hundred microns thick. Based on our understanding of human physiology, a single monolayer of tissue is not representative of an entire organ. In addition, tissue found in vivo (in the body) is within 200 µm of the closest capillary network. Beyond this distance, nutrients and oxygen diffusion is limited and tissue does not survive. The primary reason that thicker tissue constructs have not been widely demonstrated is because of the difficulty of integrating vascular networks into artificially grown tissue. We will address this challenge by using biological printing methods to assemble sophisticated capillary networks that can deliver nutrients through thick human tissue. Our four specific goals for this project include (1) development of printable biological ink, (2) coaxial printing and extrusion of a biological vessel, (3) vascular-membrane tissue characterization, and (4) perfusion studies for homogenous distribution of nutrients through multiple layers of thick tissue.
Our research could enable the first sustainable platform of networks capable of distributing oxygen and nutrients homogenously through several layers of tissue, with the use of only natural biological materials. We will address this challenge by implementing advanced printing techniques to assemble biological material into sophisticated capillary networks. The goals of this project will be achieved by developing a strong team of biologists and engineers at LLNL, as well as by forming strategic partnerships outside of the Laboratory. Our research will develop capabilities in tissue engineering of interest to the Defense Advanced Research Projects Agency, Defense Threat Reduction Agency, National Institutes of Health, and Food and Drug Administration, all of whom have expressed interest in developing organ-on-a-chip technologies to accelerate research of host–pathogen, therapeutic, and vaccine interactions with human tissue.
Mission Relevance
Integrating complex microvascular networks in thick living tissue will generate relevant tissue models that measure the therapeutic response to unknown chemical and biological agents, in support of a central Laboratory mission in national security, specifically in the area of biosecurity to develop platforms and tools for rapid medical countermeasures to emerging threats. This also supports the LLNL core competency in bioscience and bioengineering.FY15 Accomplishments and Results
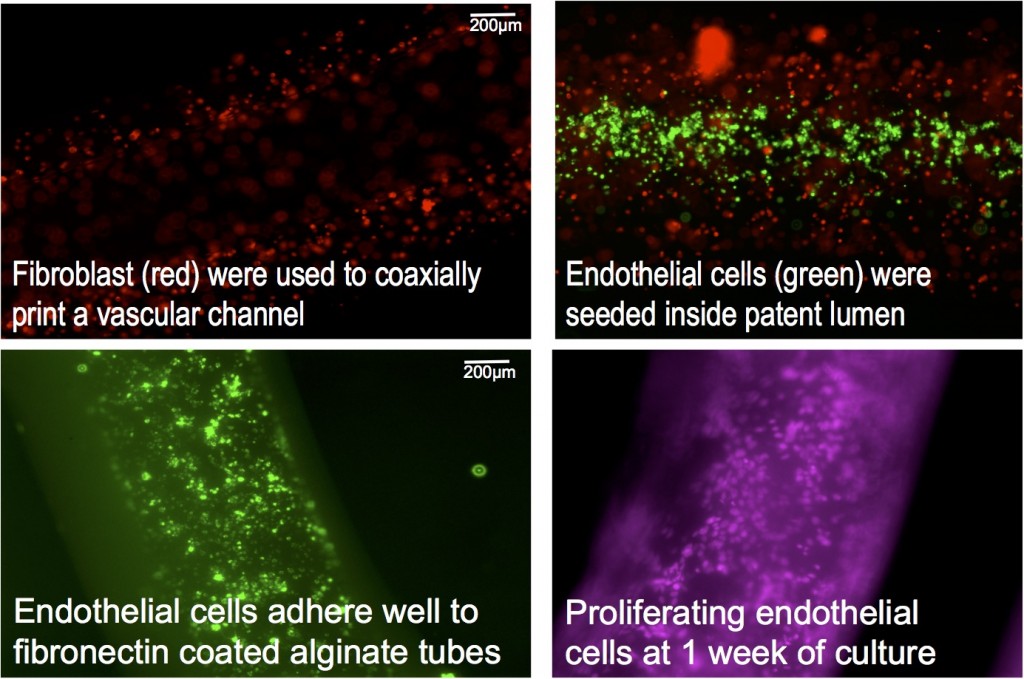
Major accomplishments for this year include (1) coaxially printing vascular-like tubes for perfusing fluids using custom-made coaxial needles—tubes printed with stromal cells (connective tissue cells for organs) in the walls had high viability immediately after printing; (2) seeding printed tubes with human cerebral microvascular brain endothelial cells that attached well and proliferated in the tubes, forming a perfused endothelial-lined vascular channel, shown in figure; (3) constructing a fluidic and housing device to connect printed tubes to external pumps to allow for perfused vascular networks; and (4) integrating printed vascular networks with our fluidic and housing device to create a larger three-dimensional tissue construct fed by perfused printed vascular networks.Publications and Presentations
- Moya, M. L., M. Cardona, and E. Wheeler, 3D bioprinted in vitro system of the microcirculation. (2015). LLNL-POST-670405.
- Moya, M. L., M. Cardona, and E. Wheeler, Bioprinting an in vitro system of the microcirculation. (2015). LLNL-PRES-674190.
- Moya, M. L., M. Cardona, and E. Wheeler, Bioprinting vascular networks for engineered tissue constructs. (2015). LLNL-PRES-676898.