Sarah Baker (17-FS-027)
Executive Summary
This feasibility study examines the use of a newly engineered bacteria strain with printed bioreactor materials to convert methane to usable fuels and chemicals. The process may ultimately reduce the cost of methane conversion by eliminating an expensive cofactor enzyme, in support of DOE's goals in science and energy.
Project Description
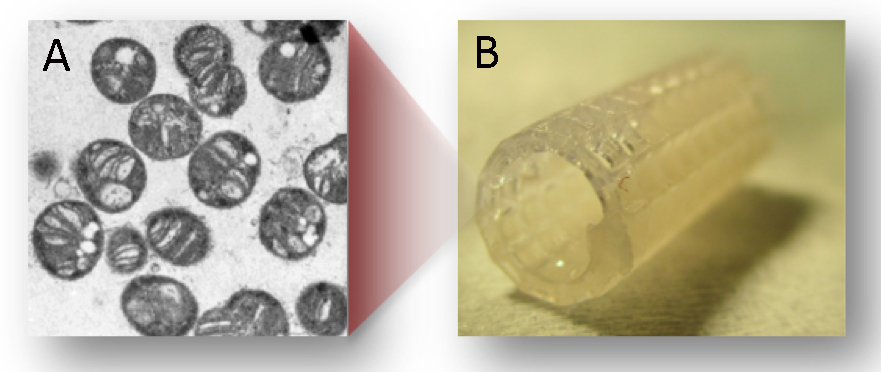
A technology to efficiently convert methane to other hydrocarbons is highly sought after as an effective way to convert “stranded” sources of methane and natural gas (sources that are small, temporary, or not close to a pipeline) to liquids for further processing. Biological methane conversion requires significantly lower energy and capital costs than chemical conversion. However, current stirred-tank bioreactors are limited by mass transfer of gas-phase reactants to the biocatalysts. Lawrence Livermore National Laboratory has developed advanced manufactured bioreactors to convert methane to other hydrocarbons. These modular and scalable bioreactors consist of printed enzyme-embedded polymer materials and are designed to make onsite biological conversion of the stranded sources of methane and natural gas efficient to collect as a liquid product suitable for fuels and chemicals. However, the necessary biocatalysts for this conversion—methane monooxygenase enzymes (enzymes that oxidize methane into methanol)—require an expensive electron donor. A possible solution may be found in a newly engineered strain of Methylomicrobium buryatense, a bacterial methanotroph that employs methane as a source of carbon and energy. We are testing the feasibility of integrating dried, whole M. buryatense with our biocatalyst materials to eliminate the need for, and expense of, the coenzyme electron donor in the methane conversion process. Ultimately, making the process more economical may increase the commercialization potential of the Laboratory’s methane bioconversion-reactor technology.
The only catalysts isolated to selectively facilitate conversion of methane gas to liquid products under ambient conditions are methane monooxygenase enzymes from specific soil microbes. There are two major hurdles to the industrial use of these enzymes: limited mass transfer of gas-phase reactants to the biocatalysts, and the requirement that each conversion of methane to methanol consumes one equivalent of an expensive cofactor of nicotinamide adenine dinucleotide as an electron donor. The bacterium M. buryatense is a methanotrophic strain suitable for large-scale production of various chemicals and biofuels. It enables the conversion of methane to lactate, a precursor to bioplastics. Because the entire M. buryatense proteome is required for the methane-to-lactate conversion, this study is focused on determining the feasibility of developing methods for incorporating the whole methanotroph proteome into a printable material while maintaining biocatalytic activity. We expect to demonstrate bench-top continuous conversion of methane to lactate without the need of the nicotinamide adenine dinucleotide cofactor. In addition, we will establish the viability of the printed bioreactor concept for methane conversion, which will have broad impact for myriad biocatalytic systems that utilize gas-phase reactants.
Mission Relevance
This study directly addresses the Laboratory’s mission research challenge in energy and resource security by helping to maintain a leadership role in natural gas conversion technologies and supports the DOE goal of a more economically competitive, environmentally responsible, secure, and resilient U.S. energy infrastructure. This study also enhances the Laboratory’s core competency in advanced materials and manufacturing by improving the tools that demonstrate the applications of advanced manufactured materials.
FY17 Accomplishments and Results
In FY17 we demonstrated continuous methane consumption of polymer-embedded methanotrophs in advanced manufactured membrane structures. The consumption rate is on par with the organisms in solution, indicating that we have created a biocompatible, printable material without major mass-transfer limitations.