Michael Nielsen | 18-ERD-055
Overview
We explored low-loss electron-energy-loss spectroscopy (EELS) characterization of liquid specimens using liquid-cell transmission electron microscopy (TEM). Unique spectroscopic features were observed in the low-loss region for some transition-metal salt solutions, and spectroscopic changes were observed upon switching between solutions. Detection limits were explored to determine the viability of EELS for chemical characterization of liquids in a complex material system. As part of these measurements, an experimental diagnostic was developed to characterize liquid-flow performance in the liquid-cell TEM sample stage. These results are now being analyzed and compiled into two or more manuscripts for submission to peer-reviewed publications.
Background and Research Objectives
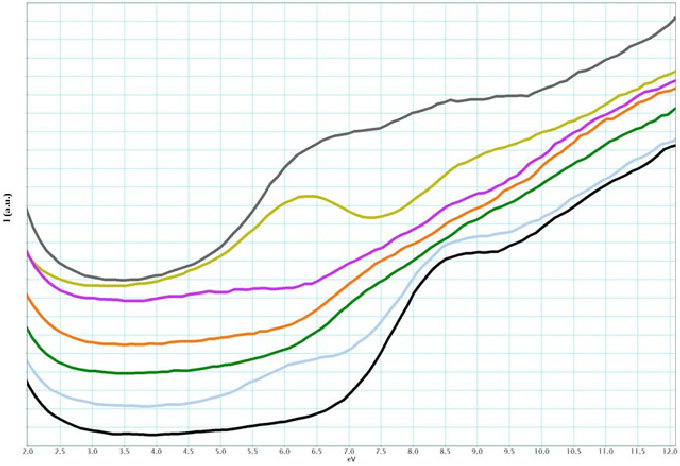
Liquid-cell TEM has been utilized in a variety of materials systems to observe the dynamics of nanoparticle nucleation, growth, and assembly and to explore on the nanoscale the performance of electrochemical devices in liquid environments. The microscope’s electron beam has been used to drive formation and growth in many of these studies of nanomaterials, while reagent flow using a plumbed TEM holder or an applied electrical bias has driven the reaction in other systems. Relatively little attention has been given to the chemical composition of the liquid phase. A spectroscopic examination of the liquid phase is almost entirely lacking, with EELS having been primarily used to determine liquid-layer thickness. To our knowledge, only a few comparisons of EELS spectra from different solvents are found within the literature. During the course of feasibility study, we observed that unique chemical signatures exist in low-loss EELS spectra obtained from some aqueous and anhydrous salt solutions, suggesting chemical sensitivity to specific metal ions and hydration of those ions, as shown in the following figure.
The primary objective of this project was to explore the spectroscopic identification of chemical signatures in solutions, which could potentially allow the probing of highly localized chemical changes at conditions relevant to real conditions in materials systems. This capability could find direct application to diverse research programs, including energy storage materials, biomimetic assembly, and accelerated corrosion.
Impact on Mission
This research supports DOE goals in science, energy, and nuclear security via a wide range of programmatic efforts at Lawrence Livermore National Laboratory (LLNL), including advanced materials and manufacturing (particularly in energy storage materials) and stockpile stewardship (by developing a new capability to investigate accelerated corrosion). Results from this project may inform the development of future research, as well discussions on the utility of purchasing a commercially available liquid-cell TEM stage for LLNL. This would be compatible with the existing Titan and CM300 microscopes and potentially find application in programs investigating energy storage materials, accelerated corrosion, additive manufacturing, and biomimetic assembly.
Conclusion
We successfully collected in situ EELS measurements of liquid samples in the TEM to demonstrate that some solutions have unique spectral features. In some cases, these features can be detected when the solutions are diluted into concentrations of 10s of millimolars. Analytical routines to extract quantitative information from the acquired datasets are being developed in conjunction with The Molecular Foundry. Additionally, a straightforward diagnostic measurement was developed to quantify the performance of liquid flow through liquid TEM sample stages. Results from this highly productive project are being synthesized into at least two manuscripts for submission to peer-reviewed publications.