Monika Biener | 17-LW-013
Overview
We explored a flow-through electrochemical reactor design for high-power-density applications, using pressure-driven mass transport of reactants through bulk porous electrodes to overcome mass-transport limitations and exploit the large internal surface of these electrode materials. The electro-catalytic conversion of CO 2 to fuels on porous Cu electrodes was chosen as a test reaction, but the flow-through electrode concept can, in principle, be applied to any other electrochemical transformation for which high power densities are desired. Preferably, the flow-through working electrode has a hierarchical pore morphology: Large pores warrant fast mass transport through the electrode at relatively low pressure differentials across the electrode, and small pores provide the high surface area necessary for achieving high conversion rates for even low reaction probabilities. Unlike flow-by electrolysis setups, the reactant stream is continuously moved through the porous catalyst electrode. Our experiments show that flowing electrolyte solution directly through the electrode increases the CO 2 reduction-related current; however, the effect is small in aqueous electrolytes, owing to the dominance of the competing hydrogen evolution reaction.
Background and Research Objectives
The CO 2 reduction reaction (CO2RR) provides a path forward to a carbon-neutral energy cycle by combining carbon capture with hydrocarbon production, if regenerative power sources such as wind and solar are used (Jhong et al. 2013). One of the main obstacles for deployment of CO2RR technology is the lack of a catalyst that combines long-term stability, high activity (low over-potential) and selectivity (ideally towards desired feedstock chemicals such as ethylene) with fast mass transport for both reactants and products (Kas et al. 2016).
Current technologies to convert CO 2 /electricity to fuels are energy- and capital-intensive. The largest plants are based on Pt catalyzed water electrolysis to convert renewable electricity into hydrogen, followed by CO 2 methanation via the Sabatier reaction or microbial fermentation. While this technology has been efficiently scaled up to industrial-sized plants (e.g., the Audi e-gas plant, with 54% efficiency using a 6 MW electrolyzer), one-step electrochemical conversion of CO 2 to high-value chemicals has the potential to significantly reduce capital and process costs.
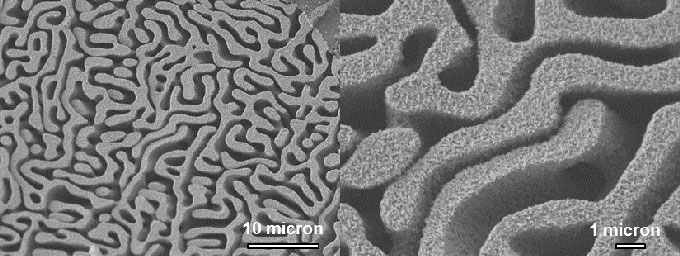
To date, copper has emerged as the best CO2RR catalyst for hydrocarbon production (Peterson et al. 2012). However, the efficiency of the direct CO 2 -to-fuel electrochemical conversion is not only determined by the intrinsic reactivity and selectivity of the catalyst material, but also the number of interactions between the reactant and the catalyst surface. The latter is determined by the electrode shape and morphology and the supply of reactants to the catalytically active surface. Current electrolysis setups typically use a flow-by operation mode with two-dimensional electrodes or surface-roughened electrodes. This has the disadvantage of exploiting only a very limited surface area of the catalyst, thus limiting the current density that can be achieved. The distinctly different length scales of the two different pore feature sizes are seen in the following figure.
Scanning electron micrographs of hierarchical nanoporous copper prepared by dealloying CuAl 2 in NaOH.
The objective of this project was to design a reactor that allows the reactant stream to flow through the catalyst material and to study the effect of reactant flow on catalytic performance. Flow-through mode operation has been studied before, but not in the context of CO 2 reduction (Saleh 2004).
Impact on Mission
This project addresses Lawrence Livermore National Laboratory's (LLNL's) energy and resource security research and development challenge by providing a pathway to efficient carbon dioxide capture via oxidative reduction to hydrocarbons using excess energy from regenerative power sources. This work also strengthens the Laboratory’s core competency in advanced materials and manufacturing by adding new material synthesis and characterization capabilities. This was the first project at LLNL on electrocatalytic conversion of CO 2 to fuels, which is now a key component of the Laboratory's carbon initiative. It provided the seed for several larger funded projects in this field, including a cooperative research and development project, a project with the Advanced Manufacturing Office, and two LDRD projects.
Conclusion
Our experiments show that flowing electrolyte solution directly through the electrode increases the CO 2 reduction-related current; however, the effect is small in aqueous electrolytes, owing to the dominance of the competing hydrogen evolution reaction. This interpretation is confirmed in that the reduction current increases significantly if the reaction is performed in ionic liquids instead of water. Our experiments reveal that, at least for our pore morphology, the beneficial effect of flow on the reduction current is mainly driven by a reduction of the diffusion boundary layer thickness. It is expected that the beneficial effect of flow on the CO 2 reduction current becomes even more pronounced if porous electrodes with a higher macropore-related surface area (smaller macropores) are used.
References
Jhong, H. R., et al. 2013. "Electrochemical Conversion of CO 2 to Useful Chemicals: Current Status, Remaining Challenges, and Future Opportunities." Current Opinion in Chemical Engineering 2 (2):191–199. doi: 10.1016/j.coche.2013.03.005.
Kas, R., et al. 2016. "Three-Dimensional Porous Hollow Fibre Copper Electrodes for Efficient and High-Rate Electrochemical Carbon Dioxide Reduction." Nat Commun 7. doi: 10.1038/ncomms10748.
Peterson, A., et al. 2012. "Activity Descriptors for CO 2 Electroreduction to Methane on Transition-Metal Catalysts." Journal of Physical Chemistry Letters 3 (2):251–258. doi: 10.1021/jz201461p.
Saleh, M., et al. 2004. "On the Effectiveness Factor of Flow-Through Porous Electrodes." Journal of Physical Chemistry B 108 (35):13419–13426. doi: 10.1021/jp048489w.
Publications and Presentations
Biener, M., et al. 2018. Flow-Through Reactor for Electrocatalytic Reactions. Record of invention IL-13253 filed on April 5, 2018.