Kyle Sullivan (16-FS-028)
Abstract
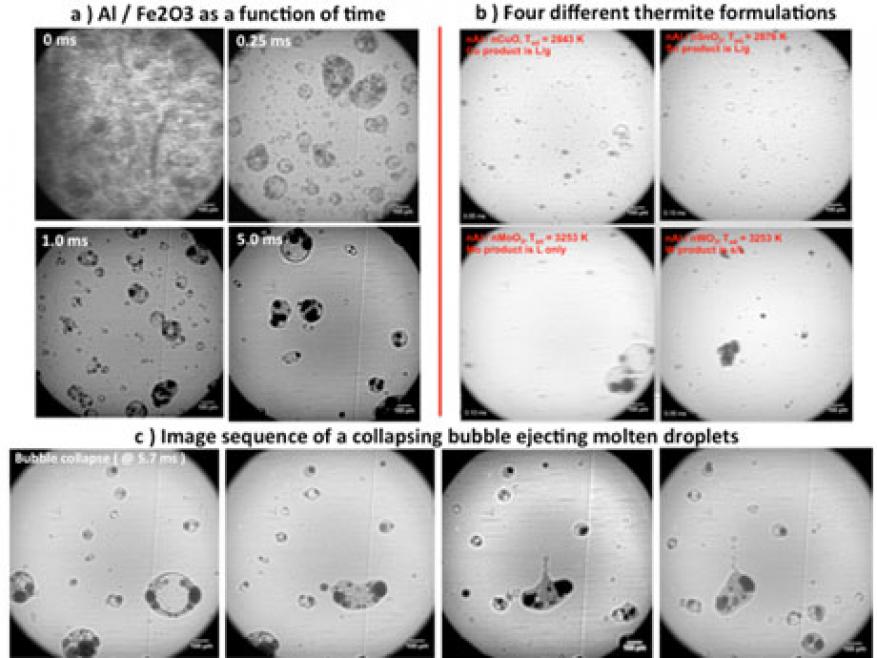
Reactive composites utilizing nanometer-scale particles have been the topic of extensive research for the past two decades. A motivating factor is that as the particle size is decreased, the mixing scale between constituents is greatly reduced, which has long thought to increase the rate of chemical reaction. While a general trend of increased reactivity with reduced size has been seen for mixtures of metal and metal oxide reactive materials (thermites), some results have demonstrated diminishing returns as the particle size is decreased. Recent results have shown that nanoparticles, which are typically aggregates of several primary particles, can undergo very rapid coalescence to form micrometer-scale particles once a critical temperature is reached. Experiments to date have been performed on very small sample masses, and sometimes under vacuum—conditions that are not representative of the environment during a deflagration. In this feasibility study, 100-mg powdered thermite samples were ignited and reacted in custom long acrylic tubes. We performed x-ray imaging at Sector 32 of the Advanced Photon Source at Argonne National Laboratory to image the particle field as a function of distance and time as the rarefied particle cloud expanded and flowed down the tube. Five different thermite formulations were investigated: aluminum and copper(II) oxide (Al/CuO), aluminum and iron(III) oxide (Al/Fe2O3), aluminum and tin oxide (Al/SnO2), aluminum and tungsten trioxide (Al/WO3), and aluminum and molybdenum trioxide (Al/MoO3), along with aluminum and copper oxide formulations with different sizes of aluminum particles ranging from 80 nm to approximate 10 mm. The results clearly show that the sample powder reacts and unloads into a distribution of larger micrometer-scale particles (approximately 5–500 mm), which continue to react and propagate as the particle-laden stream flows down the tube. This was the first direct imaging of the particle field during a thermite deflagration, and gives significant insight into the evolution of reactants to products. Analysis of phase is currently being pursued to determine whether this method can be used to deduce reaction kinetics.
Background and Research Objectives
Thermites are mixtures of a metal and a metal oxide, which exothermically react upon ignition to release chemical energy in the form of heat and/or pressure. The classical use of thermite dates back to 1904 and involved the reaction between metallic aluminum and iron oxide to produce molten iron metal to weld railroad ties together in Hungary. This method is commonly referred to as the Goldschmidt method, named after the German chemist Hans Goldschmidt. In 1995, Aumann et al. demonstrated that the reactivity in thermite formulations of aluminum and molybdenum trioxide was observed to be dramatically increased by over three orders of magnitude when nanoparticles were used in place of micrometer-sized constituents.1 Additionally, thermochemical calculations performed by Fischer and Grubelich showed that some formulations of thermite have a higher mass and/or volumetric energy density than even the nitroamine CL-20, one of the highest-power explosives used today.2 In energetic material applications, it is the rate of energy release, or power, of the chemical reaction that ultimately quantifies its performance and applications.
By using nanoparticles, it has long been thought that the power in thermites could be increased to a level in which these materials could be competitive with high-explosive applications. However, there have been several recent studies showing diminishing returns in reactivity with particle size. Researchers have suggested that a problem with nanoparticles, which are typically aggregated, is that upon melting the surface tension forces serve to drive particles to coalesce into larger particle sizes. This has been directly measured to occur in aluminum aggregates in as little as 25 ns using the dynamic transmission electron microscope at Lawrence Livermore.3 This morphology change results in a decreased surface area, and has been thought to limit reactivity. Dynamic measurements like this yield valuable information of the length scales and timescales associated with reaction. However, the measurements are difficult to make because of the fine spatial scales and timescales associated with these processes.
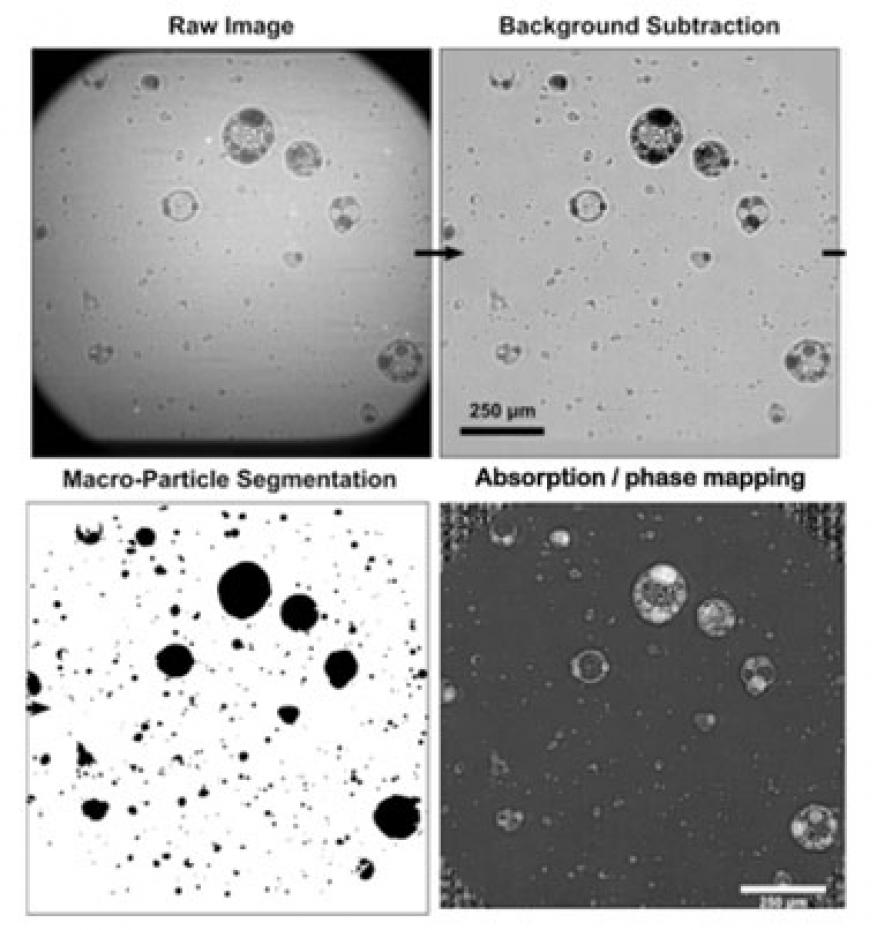
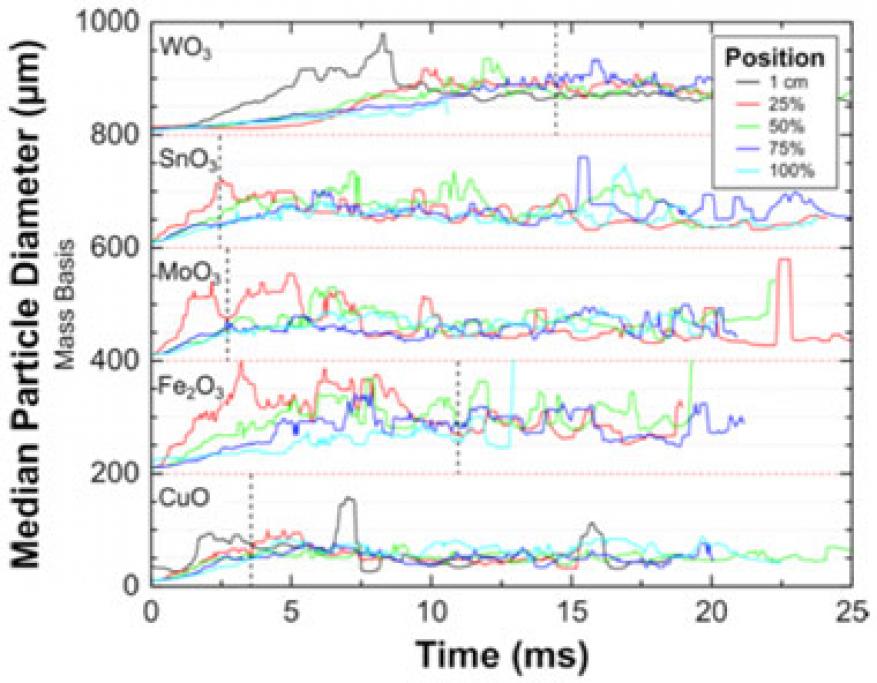
Our primary goal was to directly image the reaction of a thermite during a deflagration using the collimated x-ray beamline at the Advanced Photon Source. A 100-mg sample of thermite powder was ignited in a long, clear tube and allowed to flow in one direction down the tube as it reacted. The time-resolved particle field was then imaged perpendicular to the direction of flow, and at various distances down the tube corresponding to different times after initiation. The imaging technique allowed us to measure the unloaded particle size and, additionally, to observe phase and dynamics occurring within these particles as the reaction proceeded. The results are the very first of their kind, and significantly enhance our understanding of the dynamics occurring in thermites.
Scientific Approach and Accomplishments
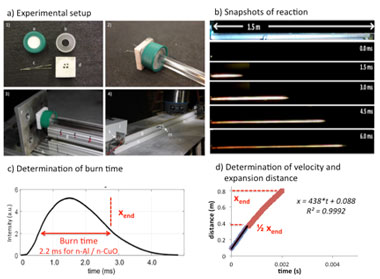
We used a Livermore-developed method known as the “extended burn tube" for testing the reactivity of thermites, as shown in Figure 1.4 A 100-mg powdered charge of a thermite is loaded into the closed end of a long, acrylic tube. An ignition wire is inserted into the powder, and resistively heated with a fast current pulse. This ignites the material, which subsequently leads to unloading and flow of a rarefied powder cloud as the material reacts and expands down the tube. It is assumed that there is a direct linkage between spatial position down the tube and reaction time. We performed x-ray imaging normal to the flow direction, and time-resolved movies of the particle field were measured. The tube could then be translated, and the experiment repeated to examine different positions. Because of the high reproducibility of the burn tube experiment, the results could be stitched together from separate experiments to draw conclusions of the overall deflagration.
Figure 1. (a) Schematic of the extended burn tube test. (b) Still shots of the reaction igniting and propagating down the tube. (c) Analysis of burn time by plotting the optical intensity versus time and measuring the full width at half maximum of the intensity. (d) Analysis of the flow velocity and quenching distance.
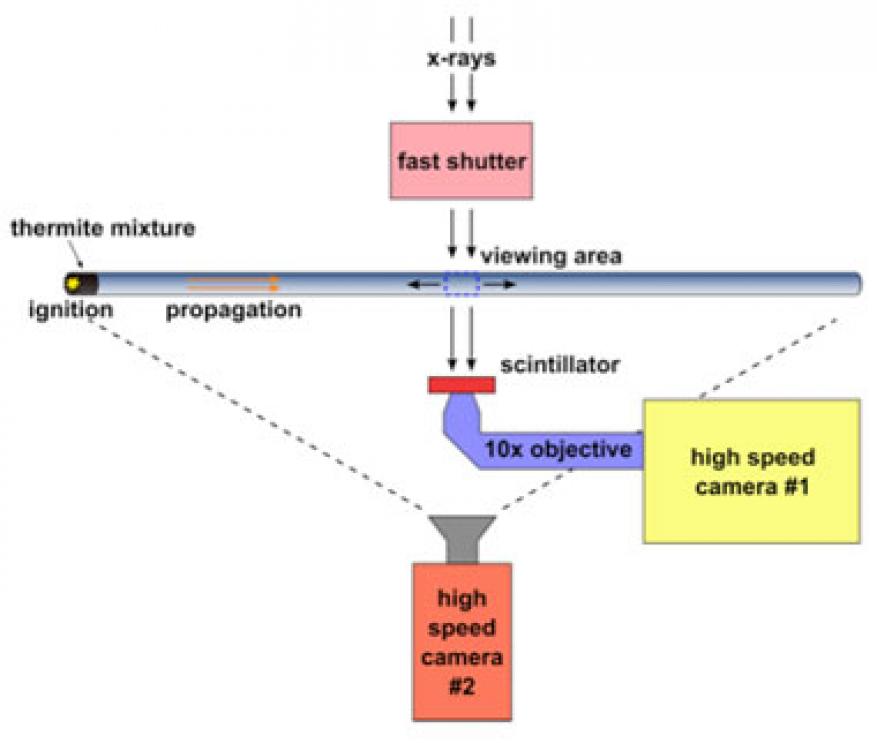
We employed the extended burn tube setup at the Advanced Photon Source twice, once in March 2016 when the beam was operating in 24-bunch mode, and once in July 2016 while the beam was in hybrid-singlet mode. In 24-bunch mode, we found that the beam intensity was insufficient to yield high-quality images from a single pulse. Instead, multiple exposures were required but, because of the high particle velocity in the tube, this led to blurring of the images. In the second visit, the beam was in hybrid-singlet mode and provided sufficient energy to image from a single pulse. A schematic of the experimental setup is shown in Figure 2. We performed the x-ray imaging perpendicularly to the flow, and a high-speed camera was used to record the luminous streak as it flowed down the tube. The tube was imaged at different positions after initiation.
Impact on Mission
Energetic materials are of interest to several programs at Lawrence Livermore, and our investigation of formulations for thermites support the Laboratory's core competency in advanced materials and manufacturing, as well as to the strategic focus area in stockpile stewardship science. Our feasibility study results can help inform the design of engineered architectures for custom energy release, and the work also applies to the development of advanced diagnostics for probing materials under extreme conditions. This work is relevant to the NNSA and Department of Defense Joint Munitions Technology Development Program, which investigates structural energetics to add functionality to munitions. Structural energetics aligns with the Livermore strength in structural materials, whose purpose is to develop structure–property relationships, including dynamic properties. Our work has the added benefit of increasing Livermore's exposure at the Dynamic Compression Sector, a new NNSA-sponsored research facility at Argonne, which will continue research on reaction of thermites to investigate the processing, ignition, and combustion characteristics of these formulations.
Conclusion
With this feasibility study we collected very-high-quality movies of deflagrating systems for the first time. This work has now transitioned to programs at Lawrence Livermore. While the exact details of the thermite reaction still remain challenging to extract from the movies, the data represents an unprecedented view of the energetic reaction and provides significant insight for future experiments. As part of future work, we plan to image thermites with different morphologies to see what ultimately controls the unloaded particle size. Phase analysis will also continue, and the goal is to determine whether the absorption coefficient within these particles can be used as a metric of reactivity. If so, this would represent a generic solution for extracting chemical kinetic parameters from composite systems, and would be a highly significant result. We have begun a collaboration with the New Jersey Institute of Technology to look at milling of custom thermites, and both the Joint Munitions Program and the Defense Threat Reduction Agency are supporting projects in FY17 for use of thermites and other reactive materials as structural energetics. Two postdoctoral researchers have been hired within the Laboratory's Materials Science Division who are, or will be, working with thermites. A third postdoctoral researcher and a graduate student employed in the materials engineering division have also recently begun examining three-dimensional printing of thermites.
References
- Aumann, C. E., et al., “Oxidation behavior of aluminum nanopowders,” J. Vac. Sci. Tech. B 13(3), 1178 (1995).
- Fischer, S. H., and M. C. Grubelich, A survey of combustible metals, thermites, and intermetallics for pyrotechnic applications, 32nd AIAA/ASME/SAE/ASEE Joint Propulsion Conf., Lake Buena Vista, FL, July 1–3, 1996.
- Egan, G. C., et al., “In situ imaging of ultra-fast loss of nanostructure in nanoparticle aggregates,” J. Appl. Phys. 115(8), 10.1063 (2014).
- Sullivan, K. T., et al., "Quantifying dynamic processes in reactive materials: An extended burn tube test." Propell. Explos. Pyrotech. 35(1),1 (2014).
Publications and Presentations
- Reeves, R. V., et al., Direct x-ray imaging of Al thermite reactions at the particle scale. (2016). LLNL-POST-693797.