Monika Biener (17-ERD-013)
Executive Summary
We are developing novel copper membranes with nanometer-scale surface features to build electrodes that will catalyze the reduction of carbon dioxide into hydrocarbons. This technology can be used as a carbon removal/mitigation strategy and advances the DOE goals of delivering tools that support a more economically competitive, secure, and resilient energy infrastructure.
Project Description
The electrochemical carbon dioxide reduction reaction provides a path toward a carbon-neutral energy cycle by combining carbon capture with hydrocarbon production, which has the potential to reduce global carbon dioxide (CO2) emissions through energy storage for intermittent renewable energy sources and green catalysis of feedstock chemicals. One of the main obstacles to deploying CO2-reduction reaction technology is the lack of a catalyst that combines long-term stability, high-activity (low over-potential) and selectivity (ideally toward desired feedstock chemicals such as ethylene) with high electrical conductivity and fast mass transport (for both reactants and products). To date, copper has emerged as the best catalyst for hydrocarbon production, and the literature describes various ways to further improve its catalytic performance. For example, oxidation–reduction cycles have been used to increase the surface area and to introduce a nanometer-structured copper surface that has been linked to enhanced activity and selectivity. However, despite increasing the area of copper foils by oxidation–reduction, the material still has a comparatively small surface area. In this project, we seek to improve the rate and energy efficiency of the CO2-reduction reaction compared to current technologies. We are developing hierarchical, porous copper membranes with a rough, ultrahigh surface area of nanometer-scale cubes, which will allow faster mass transport (i.e., flow velocity) of reactants and products through the membrane. We will test these electrodes using a novel flow-through (as opposed to the traditional flow-by) geometry, characterize their performance for hydrocarbon production, and investigate their composition and surface chemistry via advanced in situ synchrotron-based x-ray techniques.
We expect that our surface-engineered copper membranes will overcome the mass-transport and conversion limitations of current technology and lead to transformational performance improvements in the CO2-reduction reaction. We are developing and testing hierarchical porous nanometer-scale copper-cube membranes in a novel flow-through geometry for the reduction reaction. The porous structure of the copper membranes will provide significantly larger surface areas within a given footprint than typical designs with two-dimensional copper electrodes, and the hierarchical morphology enables much faster mass transport through the membrane at relatively low pressure gradients across the membrane. We expect that this combination of high surface area with the proposed novel flow-through geometry will allow us to achieve high conversion rates at low over-potentials because the geometry eliminates the need for potential-driven mass transport, and the combination of an ultrahigh internal surface area and the nanometer-scale copper-cube surface morphology enables high conversion rates even for low-reaction probabilities. Our study of the effect of ligament morphology and real-time synchrotron-based characterization will provide critical insight into the design of reduction-reaction catalysts for the CO2-reduction reaction. Additionally, our proposed copper-cube membrane has other applications such as gas-phase catalysis, super capacitors, and targets for high-energy-density physics experiments. Both the CO2-reduction reaction and gas-phase catalysis have potentially large impacts on global CO2 emissions, including CO2 capture, energy storage for intermittent renewable energy sources, and green catalysis of important feedstock chemicals.
Mission Relevance
This technology advances the DOE goals of delivering tools that support a more economically competitive, secure, and resilient energy infrastructure. This project addresses Lawrence Livermore National Laboratory's energy and resource security research-and-development challenge by providing a pathway to efficient CO2 capture via oxidative reduction to hydrocarbons using excess energy from regenerative power sources. This work also strengthens the Laboratory’s core competency in advanced materials and manufacturing by adding new material synthesis and characterization capabilities.
FY17 Accomplishments and Results
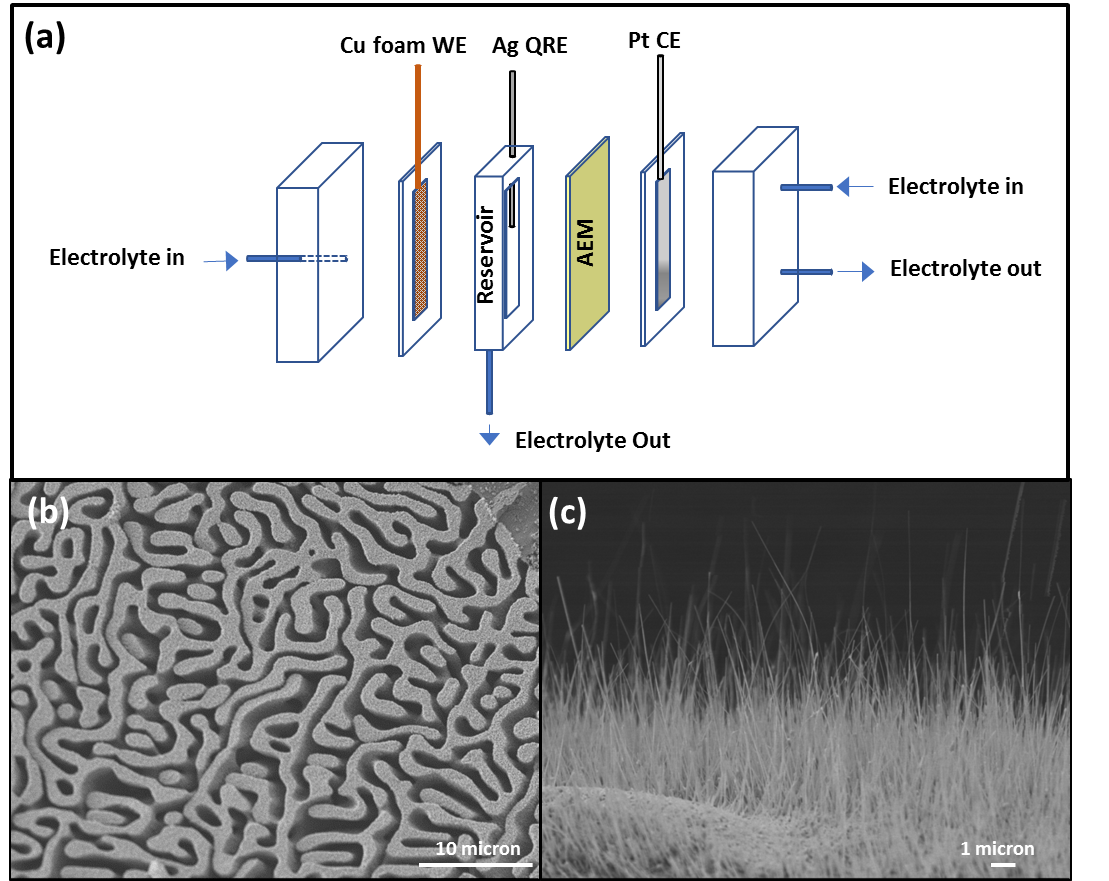
In FY 17, we have (1) successfully synthesized hierarchical nanoporous copper electrodes with different porosities by using different compositions for the starting alloys, and (2) evaluated other approaches to combine macroporosity with nanoporosity (in addition to the proposed synthesis method, one-step de-alloying), in case the robustness of the hierarchical material was not sufficient for the flow-through application. This process involved (3) growing copper nanowires on commercially available copper-foam meshes, as well as surface alloying and de-alloying copper-foam meshes with zinc. We also (4) assembled the "first-generation" flow-through cell and set up the gas chromatograph for product analysis and (5) performed electrochemical measurements on the capacity of the copper electrode.